Published online Oct 26, 2019. doi: 10.4252/wjsc.v11.i10.817
Peer-review started: June 19, 2019
First decision: August 1, 2019
Revised: August 11, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 26, 2019
Processing time: 133 Days and 23 Hours
Brain ischemic stroke is one of the most common causes of death and disability, currently has no efficient therapeutic strategy in clinic. Due to irreversible functional neurons loss and neural tissue injury, stem cell transplantation may be the most promising treatment approach. Neural stem cells (NSCs) as the special type of stem cells only exist in the nervous system, can differentiate into neurons, astrocytes, and oligodendrocytes, and have the abilities to compensate insufficient endogenous nerve cells and improve the inflammatory microenvironment of cell survival. In this review, we focused on the important role of NSCs therapy for brain ischemic stroke, mainly introduced the methods of optimizing the therapeutic efficacy of NSC transplantation, such as transfection and overexpression of specific genes, pretreatment of NSCs with inflammatory factors, and co-transplantation with cytokines. Next, we discussed the potential problems of NSC transplantation which seriously limited their rapid clinical transformation and application. Finally, we expected a new research topic in the field of stem cell research. Based on the bystander effect, exosomes derived from NSCs can overcome many of the risks and difficulties associated with cell therapy. Thus, as natural seed resource of nervous system, NSCs-based cell-free treatment is a newly therapy strategy, will play more important role in treating ischemic stroke in the future.
Core tip: In this review we compiled the latest available research regarding the use of neural stem cell therapy for the treatment of brain ischemic stroke. We discussed the benefits and limitations of this type of therapy focusing on the current efforts to improve its safety and efficacy. Further, we described a novel and clinically relevant strategy for the treatment of ischemic stroke based on cell free treatment–exosomes.
- Citation: Zhang GL, Zhu ZH, Wang YZ. Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World J Stem Cells 2019; 11(10): 817-830
- URL: https://www.wjgnet.com/1948-0210/full/v11/i10/817.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i10.817
Globally, stroke is one of the top three common causes of death and disability. It is divided into the following two types: Ischemic and hemorrhagic stroke, of which ischemic stroke accounts for more than three-quarters of cases (about 80%-85%)[1]. Following stroke onset, patients can suffer from various neurological dysfunctions that renders them unable to take care of themselves. This seriously affects their quality of life, impacts on their patients and their families physically and mentally, as well socially and economically. Recently, numerous preclinical and clinical studies[2,3] have discovered many drugs or molecules that may potentially exert certain beneficial effects in the treatment of ischemic stroke; however, these drugs exhibit limited or no therapeutic efficacy under clinical applications; thus, a novel drug for this purpose still needs to be explored.
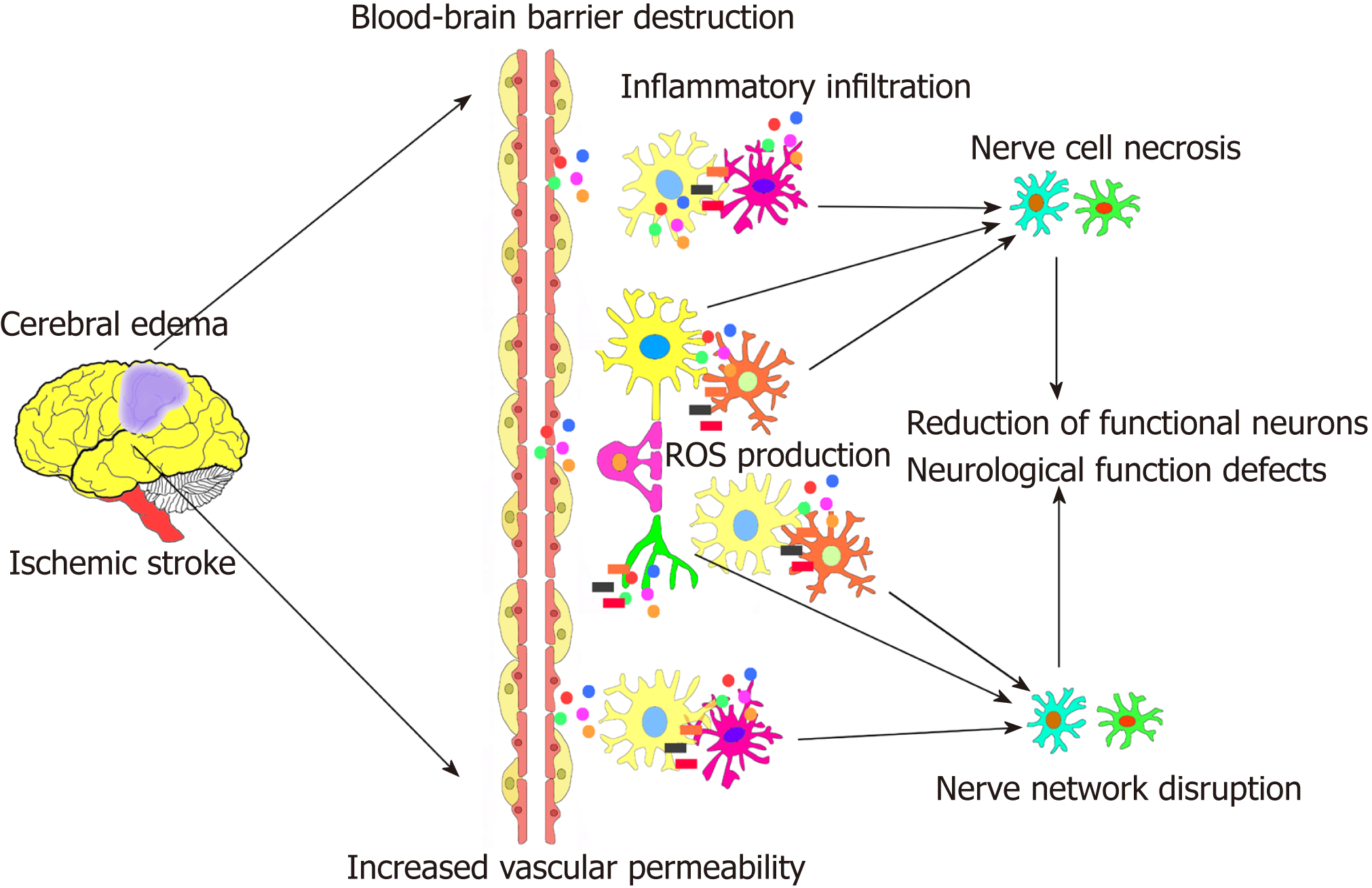
Ischemic stroke can induce some harmful pathophysiological changes around the ischemic area of the brain[4-6]. This event is mainly characterized as acute ischemia and hypoxia of nerve tissue after blood interruption to local brain tissue in a short period of time. This is followed by a series of cascade reactions, including the production of local oxygen free radicals and reactive oxygen species, increase in local tissue permeability, generation of cerebral edema, local immune inflammatory cell infiltration, development of neuroinflammation, and blood-brain barrier destruction. These lesions significantly expand in a short period of time, leading to local nerve cell damage and necrosis, reduction of functional neurons, and axon network disruption. Subsequently, due to the extremely poor microenvironment around the peri-ischemic cell regions, secondary brain damage occurs, resulting in an irreversible neural tissue injury. The limits self-repair of nerve tissue. Even after recovery of blood flow to the ischemic area, enough nerve cells and an appropriate immune microenvironment to supplement and repair the functional nerve tissue that died are still not available[4-6]. This eventually leads to permanent local nerve tissue loss and nerve function defects of the brain (Figure 1).
However, the treatment of brain ischemic stroke is individualized and involves heterogeneous approaches, most of which are closely related to the location of ischemia, the age of patients, and the ability of local nerve self-repair. The main purpose of clinical treatment of stroke is to restore local cerebral blood perfusion and reduce the occurrence and degree of disability or dysfunction after stroke onset as early as possible[2,7]. Due to the weaker regeneration ability of endogenous nerve cells and the harmful effects of an ischemic microenvironment, there are currently no effective methods or strategies available for treatment. Recombinant tissue plasminogen activator (r-tPA) is the only effective class 1A recommended drug for the treatment of acute ischemic stroke approved by the American Food and Drug Administration, but it has a very narrow treatment window (generally within 4.5 h, and no more than 6 h) and it has various treatment-related contraindications or complications (such as increased risk of cerebral hemorrhage)[8-10]. Therefore, its application has been markedly limited; only a small percentage of patients (no more than 5% of patients) can receive this timely and effective treatment. In addition, intravascular interventional therapy (such as endovascular thrombectomy) can be used as an adjuvant or replacement therapy for early reocclusion of large vessels in patients with contraindications or complications of intravenous thrombolysis[11-13]. This adjuvant therapy can extend the time window of potential treatment to 12 h, and it can also improve the functional independence and vascular remodeling rate of the patient compared to tPA treatment alone. However, the treatment time still has an important effect on the efficacy of this intravascular treatment, because just a 30 min delay beyond the timeframe can significantly reduce the functional independence of patients. Moreover, current endovascular interventions are still difficult to generalize, since only regional stroke centers with neurological intervention capabilities can perform this operation, and only less than 10% of stroke patients are eligible to undergo this effective treatment[11,14].
Thus, treatment of subacute and chronic phases of stroke is important, and it is mainly based on the clinical rehabilitation of patients with symptoms (such as different rehabilitation methods according to different stages and dysfunctions)[2,7,15-17]. This includes subject stroke unit nursing, home or hospital rehabilitation training, task-oriented training, mandatory exercise therapy, different high-intensity training, repetitive task training, spatial sensation, and language ability training. Although the abovementioned rehabilitation methods can improve the symptoms of some patients to a certain extent after long-term adherence, the effect of rehabilitation is limited, and it is still not enough to improve the overall prognosis of stroke patients. Therefore, the therapeutic prognosis of patients with brain ischemic stroke is closely related to the permanent loss of local functional neurons and networks, the repair of local glial scars, and the lack of activated endogenous neural stem cells[7,18-20].
In 1992, Reynolds et al[21] isolated a cell population with self-renewal abilities and a multi-directional differentiation potential from the striatum of adult mice; these were proposed as neural stem cells (NSCs). Subsequently, in 1997, Mckay et al[22] officially defined the concept of NSCs as a self-renewing cell population with pluripotent abilities that can differentiate into neurons, astrocytes, and oligodendrocytes. Recent studies[23,24] have confirmed that NSCs are present in the lateral ventricle (sub-ventricular zone, SVZ) and hippocampal dentate gyrus (subgranular zone, SGZ) of adult animals. NSCs in the SVZ region mainly migrate along the rostral migratory stream to the olfactory bulb, whereas stem cells in the SGZ migrate to the granule cell layer, and finally differentiate into various neural cells and integrate into nerve networks[23-26]. In addition, with the exception of SVZ and SGZ, the human brain possibly consists of another stem cell pool in the deep ventral region of the prefrontal cortex, due to the highly developed prefrontal lobe in the human brain.
Furthermore, endogenous NSCs exist in vivo, whereas exogenous NSCs are cultured in vitro. Under normal conditions, endogenous NSCs in the body are in a static, undifferentiated dormant state (called quiescent NSCs, qNSCs), and maintain a dynamic balance in the stem cell pools[27-29]. Once they are exposed to external stimuli such as brain damage, qNSCs can be activated to proliferate, migrate, and differentiate, thereby participating in the repair process of damaged nerve tissue[29-31]. Usually, the number of endogenous cells activated is very limited, and a large proportion normally differentiates into glial cells. Glial scars are involved in tissue repair, so that the loss of functional neurons (neural network) is not enough to supplement[32-34]. Moreover, significant neurological dysfunction still persist for a long time after brain injury, indicating that the neuroregenerative ability of endogenous cells is largely insufficient, and is not enough to replace damaged functional nerve tissue[7,35,36].
With the development of stem cell therapy research, adult stem cells have received more and more attention and provide new directions for future clinical treatment of refractory diseases including ischemic stroke[37-40]. NSCs have many advantages, such as self-renewal, low immunogenicity, and good histocompatibility, as well as multi-directional differentiation potential; they can differentiate into three types of nerve cells to maintain and repair damaged brain tissue. Thus, NSCs act as a natural active resource and are considered to be a good tool for treating nervous system diseases. Numerous preclinical studies[39,40] have found that transplantation of exogenous NSCs can significantly complement or replace damaged tissues and treat various neurological diseases. Studies[36,41-44] have also shown that after treatment of neurological diseases with exogenous NSC transplantation, the deficiency of endogenous NSCs was not only supplemented in vivo, but the immune micro-environment around the tissue injury area was also improved. Many preclinical studies[45-47] have demonstrated that exogenous NSCs have a certain therapeutic effect in neurological diseases, and certain studies have found that the therapeutic functions of exogenous NSCs can be enhanced by combining or overexpressing them with some cytokines [such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), or NGF etc]. However, the efficiency of exogenous stem cell transplantation therapy is still controversial, mainly due to the low grafting efficiency of exogenous stem cells in the brain (less than 5%). Furthermore, the inflammatory immune microenvironment is very severe. Nowadays, the mechanisms of treatment with stem cell transplantation are not entirely clear, and further research methods are needed.
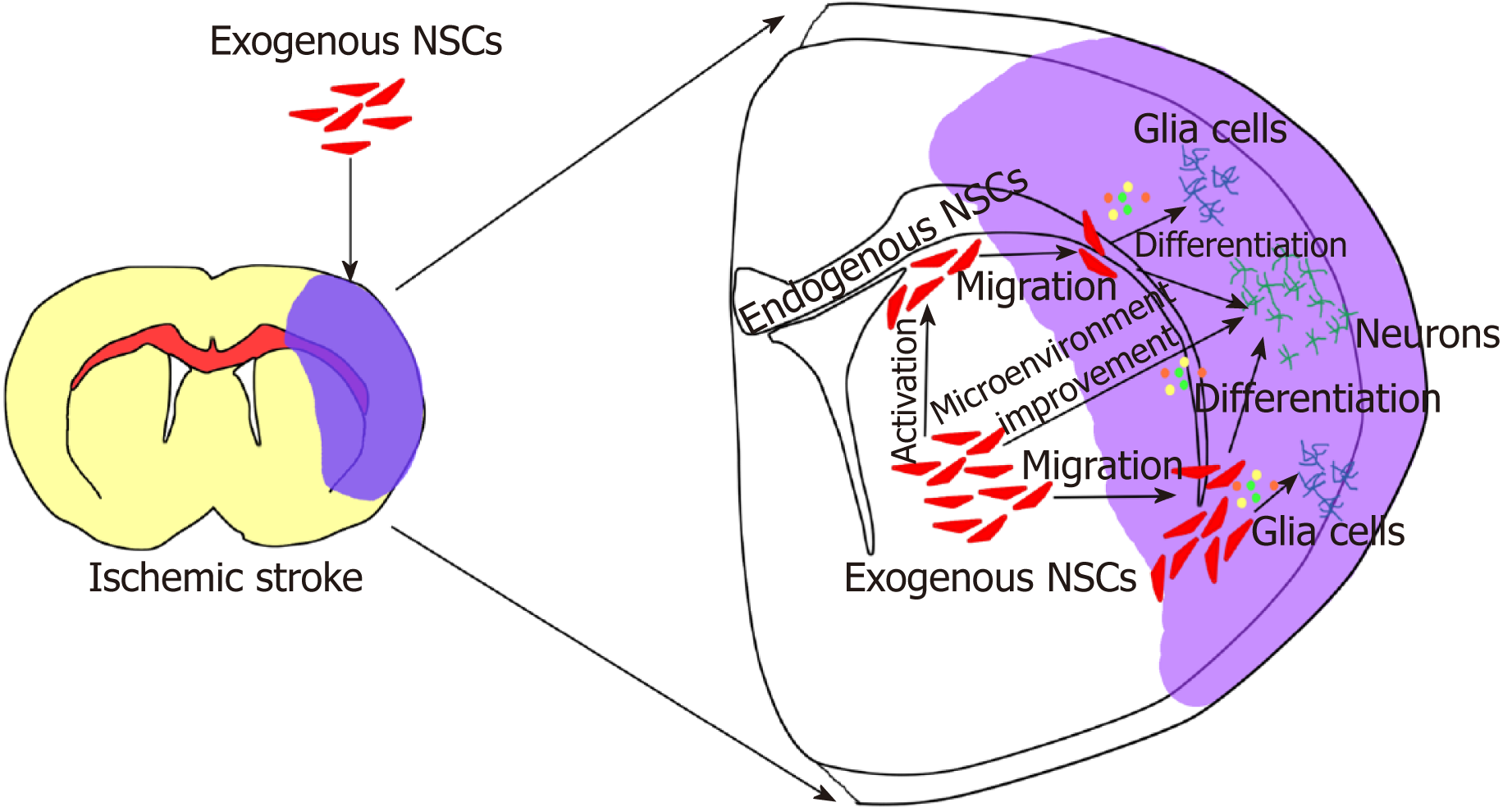
Due to the ethical, therapeutic efficacy, and safety issues, exogenous NSC trans-plantation therapies have a long way to go to reach the stage of clinical application. At present, only a few clinical studies have been conducted; however, a number of preclinical animal experiments have been conducted[38,40,48-50]. A large number of these studies[37-40] have evaluated the therapeutic efficacy and safety of transplanted exogenous NSCs in preclinical animals with brain ischemic stroke. Their results demonstrated that exogenous NSCs could significantly improve the prognosis of cerebral ischemic animals, and not only the functional outcome was improved, but also the histological infarct volume was significantly reduced, with no obvious security issues. The following two main mechanisms have been approved for the activity of exogenous NSCs in the treatment of brain ischemic stroke[36,41-44,51,52] according to the first mechanism, transplantation of exogenous stem cells compensate for the deficiency of endogenous stem cells and activate more endogenous cells to repair the nerve damage; the second mechanism reveals that transplantation of exogenous stem cells improves the inflammatory immune microenvironment around the ischemic regions, which then mediates neural network reconstruction based on the bystander effect (Figure 2).
Currently, the results of NSC transplantation for brain ischemic stroke in animal models are satisfactory. Moreover, the efficacy and safety of stem cell transplantation has also been confirmed. Lees et al[53] and Vu et al[54] used meta-analyses to evaluate the therapeutic efficacy of stem cell transplantation (including NSCs) in 117 and 46 preclinical animal models with cerebral ischemic stroke, respectively. After treatment, the neurological function of cerebral ischemic animals is improved significantly, and the volume of cerebral infarction reduced. Furthermore, the degree of prognosis improvement was correlated with the source of stem cells, injection route, injection timing, and dose of injection[53,54]. Chen et al[40] collated and analyzed animal studies of NSC therapy for the treatment of brain ischemic stroke. A total of 37 studies and 54 independent intervention groups were analyzed and meta-analyzed. The results showed that transplantation of NSCs significantly improved neurological function and histological structure outcomes of cerebral ischemic animals. Of the studies analyzed, 36 reported neurological improvement, 22 reported improved histology, and 21 reported beneficial outcomes in both neurological function and histological structure. They also found that the degree of improvement in prognosis function of ischemic animals had a certain correlation with the injection time of NSCs, the source of stem cells, and whether immunosuppressive agents had been used[40]. No significant safety problems were found. Although some differences in research quality and different degrees of publication bias between the different animal experiments exist[55-59], the overall results suggest that NSCs can effectively improve neurological function of cerebral ischemic stroke animals. They can reduce the area of ischemic infarction, proliferate, migrate, and differentiate into neurons in vivo. In addition, some neurons may be integrated into the neural network of the brain.
At present, there are very few clinical trials with NSCs registered in the Clinical Trial Database (ClinicalTrials.gov) for brain ischemic stroke. The first study is a Phase II clinical trial (NCT02117635) conducted at the Queen Elizabeth University Hospital of the University of Glasgow, UK. The current status of the trial shows that it has been completed, but no publications have been made for the Phase II clinical trial to date. They published the Phase I clinical trial (NCT01151124) of human NSC therapy for chronic cerebral ischemic stroke in the Lancet issue from August 2016[38]. This clinical study was an unblinded, single-center transplantation study that involved different doses of CTX0E03 cells (an immortalized NSC line, ReNeuron). Allogeneic transplantation of immortalized NSCs CTX0E03 was performed by stereotactic injection. A total of 11 patients were enrolled. Four groups were injected with different doses of exogenous NSCs. The patients were followed up for 2 years and evaluated by the NIHSS (National Institute of Health stroke scale) and Barthel Index methods. All of the evaluated methods and MRI images confirmed that the exogenous NSCs significantly improved the neurological function of patients after NSC transplantation, with no related adverse reactions[38]. This study was the first exogenous NSC transplantation performed in clinical patients with brain ischemic stroke[38]. None of the patients used immunosuppressants due to human leukocyte antigen (HLA) mismatch, and the results showed that some patients had significant neurological improvement. However, because the number of patients included in a Phase I trial are small, and no control or placebo groups are enrolled (which may relate to ethical issues), more detailed data in the Phase II clinical trial are expected. The second study is a Phase I clinical trial registered with Beijing Bayi Brain Hospital (NCT03296618). In this study, Exogenous NSCs were used from Neuralstem’s NSI-566 cell line derived from primary human fetal spinal cord tissue. The results of this Phase I clinical trial have not yet been published.
Although preclinical studies have confirmed the efficacy and safety of NSC transplantation for the treatment of ischemic stroke, there are still some controversies. Since the grafting efficiency or survival rate of stem cells is less than 5% in vivo, there are still many problems that should to be addressed before this treatment can be used for clinical applications[60-63]. The most important issue is the grafting efficiency and differentiation ratio of NSCs in vivo after transplantation. In addition, both endogenous and exogenous NSCs differentiate into glial cells in vivo in a significantly higher ratio than that of neurons[64-66]. Thus, many studies have attempted to modify the gene expressions or protein levels of NSCs using different strategies such as virus transfection to express specific genes, pretreatment of cells with inflammatory immune factors, and combination with cytokines to increase the therapeutic effects of transplanted cells.
BDNF can promote the differentiation of transplanted NSCs into neurons and increase their survival[67,68]. Therefore, studies have attempted to overexpress the BDNF gene in NSCs for improving the therapeutic potential of stem cells in vivo[67,68]. After transplantation of human NSCs that overexpress the BDNF gene in the ischemic striatal region of MCAO rats, the contralaterally transplanted NSCs were found to migrate to the infarcted area via MRI images. Neurobehavioral functions of ischemic rats were also significantly improved, and the transplanted cells co-localized with Nestin, DCX, and MAP2 positive cells, indicating that the transplanted NSCs participated in nerve regeneration and functional recovery in vivo. Lastly, protein expression of BDNF was also high in the ischemic regions[46].
Neurotrophin-3 (NT-3) belongs to a family of neurotrophic factors and has been found to be involved in mediating stem cell survival and inducing neural differentiation[69,70]. The lentiviral vector (LV) encoding human NT-3 was constructed and transfected into NSCs, and then transplanted into the ipsilateral striatum region of MCAO rats. After 2 to 4 wk of transplantation, secretion of NT-3 protein was significantly higher than that in the control group. Concurrently, the neurobehavioral function in cerebral ischemia rats was significantly improved, when compared to the control group[71].
VEGF is an important angiogenic factor, and it is involved in mediating angiogenesis and nutrient supplementation[72,73]. After transplantation of NSCs transfected with the VEGF121 gene into the ischemic surroundings of stroke rats, studies found that the cells survived and migrated in the ischemic area for up to 12 wk after transplantation. At the same time, the neurological function of the cerebral ischemia rats was significantly improved, compared to the untransfected NSC group, indicating that VEGF121 transfection additionally increased the therapeutic efficacy of transplanted NSCs[74].
The above studies suggest that exogenous genes can be introduced into NSCs by LVs. The modified NSCs carry the therapeutic-related genes to the damaged areas and can express them effectively, and finally increasing the repairing effects of transplanted NSCs.
Although overexpression of genes in NSCs leads to better transplantation outcomes by promoting trophic or survival signaling to cells, more beneficial, simpler, and safer methods need to be developed for future clinical applications. Consequently, studies have introduced immunological correlations where NSCs are pretreated with cytokines or inflammatory factors for treatment[75,76].
Interleukin-6 (IL-6) is a pro-inflammatory cytokine involved in the pathogenesis of various neurological diseases including stroke[77,78]. Serum IL-6 levels in patients with clinical ischemic stroke are associated with poor infarct volume and long-term prognosis[79]. When NSCs were pretreated with IL-6, results showed that stem cells were reprogrammed, and the signal transduction and transcription activator 3-mediated manganese superoxide dismutase (SOD2) was significantly up-regulated in the cells[60]. Expression of SOD2 promoted cell survival in the ischemic area; pretreated stem cells also induced the secretion of VEGF, promoted microvascularization, and significantly reduced the infarct size of cerebral ischemia, as well as improved neurological functions. These results demonstrated that pretreatment with IL-6 can properly reprogram NSCs to withstand the oxidative stress environment after an ischemia-reperfusion injury, induce angiogenesis, and ultimately improve the effectiveness of transplanted stem cells for the treatment of ischemic stroke[60].
In addition, expression of inflammatory cytokines such as IL-1, IL-15, and Interferon-gamma (IFN-γ) normally increase after central nervous system injury. When NSCs were pretreated with anti-inflammatory factors such as IL-4 or IL-10, the results were consistent with those using a pretreatment of IL-6. These cytokines mainly played a neuroprotective role and promoted the migration of cells to the injury site. The pretreatment method dominantly provided a more favorable microenvironment for the proliferation of NSCs after transplantation[80,81]. Additionally, BDNF-pretreated with NSCs produced similar results, where increased cell survival, migration, and improved neurological function were seen in cerebral ischemic animals after transplantation[82].
Thus, a better understanding of the relationship between neuro-inflammation and neurogenesis, and an understanding of the potential mechanisms of inflammatory stimulation in cerebral ischemia is essential. In vitro pretreated stem cells with cytokines or inflammatory factors may further induce the migration of NSCs to inflammatory regions, increase the neuroprotection of NSCs, and more effectively increase the therapeutic effects of stem cells.
Cytokines can regulate the self-renewal, proliferation, and differentiation of stem cells, but to maximize the therapeutic potential of stem cells and ameliorate the damage, regulation of the microenvironment may be crucial. Currently, the main direction of NSC-based research is to explore new tools for nerve regeneration. Viral vectors and gene therapy may have certain deficiencies, such as potential tumor formation and lack of efficiency. Studies[81,83,84] have attempted to deliver therapeutic drugs through implanted pumps for sustained release, but deficiencies still persist with these strategies.
Neurotrophic factors can increase the survival of NSCs and promote their proliferation or differentiation. VEGF plays various roles in the CNS, including pro-angiogenesis, neurogenesis, and neurotrophic and neuroprotective effects[73,85,86]. Study have attempted to investigate the feasibility of co-administration of VEGF with human NSCs[87]. The results showed that VEGF and transplanted NSCs had a certain synergistic effect in cerebral ischemia. The combination-treatment group expressed a better behavioral recovery than single-treatment groups, and the degree of brain atrophy in the cerebral cortex and striatum was significantly decreased. However, the distribution of VEGF was not co-localized with NSCs, suggesting that VEGF promoted the therapeutic efficacy of NSC transplantation through pro-angiogenic effects[87]. IFN-γ is a mediator of the pro-inflammatory pathway and plays an important role in the ischemic brain. However, IFN-γ does not hamper the ability of NSCs in vitro. Study[88] have shown that co-delivery of IFN-γ (50 ng) enhanced the effects of transplanted NSCs in ischemic rats. The study found that in the combination-treatment group, neurogenesis was significantly increased when compared to other groups in vivo. Moreover, co-treatment with IFN-γ and NSCs provided additional beneficial neurological outcomes. Thus, low concentrations of IFN-γ can mediate NSC functions and facilitate their ability for neurological repair[88].
In addition, vascular progenitor cells (VPC) was co-transplanted with NSCs. It was found that co-transplantation of NSCs and VPC enhanced the differentiation ratio of neurons and microvessel formation in vivo; furthermore, it significantly improved motor function and reduced the infarct volume in rats with cerebral ischemia[44]. Thus, co-repair of nerve and blood vessel may be more effective[44]. Moreover, the combination of BDNF and NSC transplantation resulted in enhanced therapeutic effects when compared to transplantation of NSCs alone[89]. However, the combination of these cytokines with cells may have some unavoidable problems such as dosage and injection methods; thus, a safe dosage must be first established to avoid harmful side effects, and secondly, a proper balance between cytokines and cells should be determined.
Although both preclinical and clinical studies have confirmed that transplantation of exogenous NSCs can treat various refractory nervous system diseases such as ischemic stroke and neurodegenerative diseases, there are still some limitations and potential side effects including a large-scale production bottleneck of stem cells, potential allogeneic rejection of cells, risk of cell tumorigenicity, grafting or survival efficiency of cell transplantation, difficulties with the administration route of cells, and targeting problems. All of those limit the rapid clinical transformation and application of NSCs.
The first limitation pertains to the source of NSCs. Given that they only exist in a specific stem cell pool of the brain[22,25], it is difficult to obtain a large number of homogenous cells in vitro. Additionally, other limitations in procuring NSC also exist such as the gradual aging of culture cells, repeated extraction and infusion of cells, cost, safety, and ethical issues. Secondly, the obtained exogenous stem cells should be allogeneic, a potential obstacle in the application of SCs is immune-rejection after cell transplantation[90,91]. Similar to any tissue or organ transplantation, allogeneic stem cells can be rejected by the host immune system. The main reason for this is that the transplanted tissue does not match the HLA in the host. Typically, host T lymphocytes recognize MHC class 1-protein antigens on other cells, if they do not match, the immune system is activated and begins to attack the transplanted cells. Although the brain has historically been considered an immune privileged area, current studies have found that immune cells can also produce immune rejection in the brain[92,93].
Furthermore, the most prominent problem of cell therapy is the potential tumorigenicity of transplanted exogenous stem cells. Stem cells, especially embryonic stem (ES) cells or induced pluripotent stem (iPS) cells, are pluripotent cells that may form teratomas or malignant tumors when implanted into a living host[94,95]. Studies have found that iPS cells derived from B6 mouse embryonic fibroblasts undergo immunological rejection after transplantation into B6 mice and also produce teratomas. ES cells derived from B6 mice also produce teratogenesis in mice, even though no significant immune rejection occurs[95,96]. Simultaneously, the tumorige-nicity of iPS cells is also related to the mutagenesis of the c-Myc gene insertion site and the persistent expression of reprogrammed exogenous genes[96-98]. A small number of contaminated exogenous cells may also induce tumorigenesis in allogeneic transplantation, and even very small amounts of contaminating undifferentiated ES cells have been found to produce tumors in nude mice[99]. Therefore, the safety regarding the use of pluripotent cells cannot be ignored. Although NSCs have not yet been found to be tumorigenic, their abnormal proliferation after in vivo transplantation may lead to tumor formation.
Finally, a series of cascades following cerebral ischemia, neuro-inflammation, and immune responses can severely affect the survival of cells after transplantation, reduce their ability of replacing damaged neurons, and eliminate the therapeutic effect of cell transplants[80,90,100]. In addition, inflammatory factor could promote glial differentiation of NSCs, resulting in the generation of GFAP-positive cells. And the immune response produced after cell transplantation may also facilitate NSC differentiation into glial cells. This suggests that inflammation may inhibit neuronal generation. However, stem cell-based therapies can modulate the host inflammatory response to recreate a favorable cellular microenvironment and prevent further endogenous cell death. Through regulation of immune inflammation, transplanted cells can increase the chances of endogenous cell survival, but the mechanisms are not clear and need to be further explored.
Currently, stem cell-based therapy is the most promising method for the treatment of refractory diseases. A large number of studies[38,40,48-50] have confirmed the effectiveness and feasibility of stem cell therapies for refractory neurological diseases including ischemic stroke. Although transplanted exogenous NSCs can provide neuroprotective effects after acute stroke and supplement lost nerve tissue for chronic stroke (through direct cell replacement and enhanced endogenous repair), the ultimate goal of complete recovery is not reached, and there are still some problems that need to be resolved.
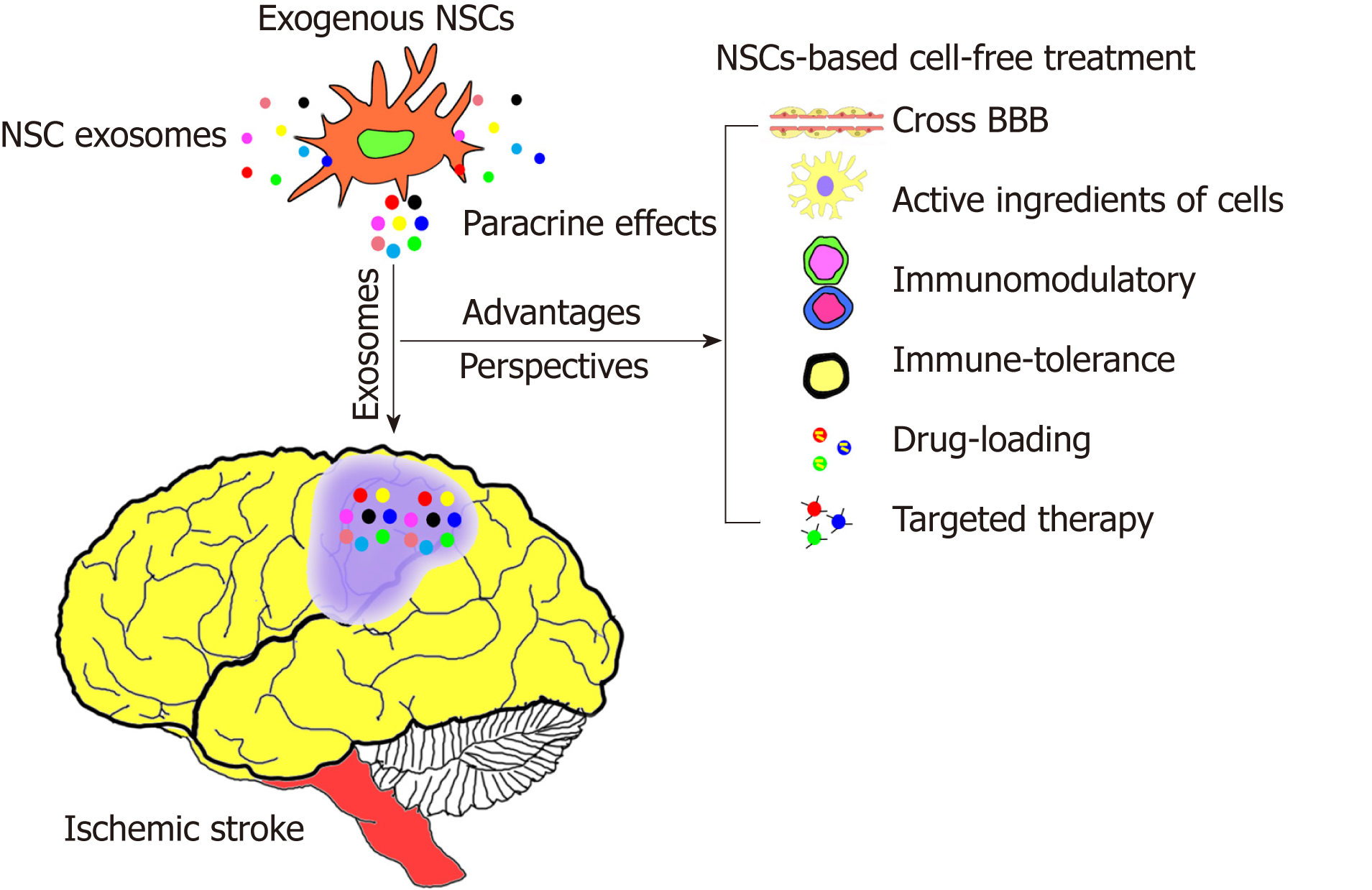
Several studies[101,102] have confirmed that the paracrine effect plays a major role in stem cell transplantation, and the main molecules responsible for this paracrine effect are extracellular vesicles (EVs). Exosomes are small molecules of exocytic vesicles derived from cells that play a major role in EVs. Exosomes are about 30-150 nm in diameter and can be secreted by all cells including proteins, lipids, and RNAs; furthermore, they play a very important role in cell-to-cell communication[103-105]. Since exosomes, in part, possess active ingredients and functional properties of the cells from which they are derived, they can be used to develop a new type of cell-free treatment. Relevant preclinical and clinical studies[106-108] have confirmed that exosomes derived from stem cells are safe and effective; not only can they cross biological barriers (such as the blood-brain barrier, BBB), but they also exhibit immune tolerance and are relatively stable (no immune rejection) in vivo. At the same time, the tumorigenicity of exogenous cells can be avoided (due to the cell-free treatment). In addition, they can be modified by nanomedicine technologies to enhance their therapeutic effects, and facilitate the route of administration or the targeting of treatment[106-108].
Exosomes have been developed as early diagnostic markers for some diseases (such as tumors) and are developing into novel molecules with potential targeted therapeutic effects. Although exosomes were discovered 30 years ago, their clinical relevance has significantly increased in recent years[107-109]. Furthermore, there is growing evidence demonstrating that exosomes are critical for the benefits of cell therapy[106,110-113]. Since exosomes can overcome many of the risks and difficulties associated with cell therapy, they can be developed as a new strategy to replace stem cell therapy (stem cell-based cell-free treatment method)[101,110,111,114-117]. However, the therapeutic potential and mechanism of exosomes in the nervous system, especially NSC-derived exosomes, has not been studied extensively. For example, the dynamic migration and kinetics of transplanted exosomes derived from NSCs in vivo, the type of exosome acting on cells, and the action mode with the local immune micro-environment need to be further studied. Moreover, the specific components of exosomes (such as proteins or miRNAs or lncRNAs) that have a significant potential of action are also unknown and need to be further explored.
In conclusion, currently the most promising treatment approach for refractory neurological diseases including ischemic stroke is based on stem cell transplantation (Figure 3). As a special type of stem cells that are present only in the nervous system, NSCs play a very important role in repairing neurological diseases. NSCs are a type of natural seed resource that not only can supplement necrotic nerve cells or tissues, but also participate in endogenous repair mechanisms. Furthermore, exosomes derived from NSCs have similar functional properties, and may serve as a new research topic in the field of stem cell research. Thus, using stem cell-based cell-free treatment, exosomes can be developed as a new therapeutic strategy, and they may play a more important role in the future.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Wakao H S-Editor: Zhang L L-Editor: A E-Editor: Xing YX
| 1. | Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2522] [Cited by in RCA: 2679] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 2. | Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1594] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 3. | Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339-1347. [PubMed] |
| 5. | Weinstein JR, Koerner IP, Möller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1212] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 7. | Hankey GJ. Stroke. Lancet. 2017;389:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 891] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 8. | Ning M, Sarracino DA, Buonanno FS, Krastins B, Chou S, McMullin D, Wang X, Lopez M, Lo EH. Proteomic Protease Substrate Profiling of tPA Treatment in Acute Ischemic Stroke Patients: A Step Toward Individualizing Thrombolytic Therapy at the Bedside. Transl Stroke Res. 2010;1:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364-2372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 808] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 10. | Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W; Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1556] [Cited by in RCA: 1857] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 11. | Badhiwala JH, Nassiri F, Alhazzani W, Selim MH, Farrokhyar F, Spears J, Kulkarni AV, Singh S, Alqahtani A, Rochwerg B, Alshahrani M, Murty NK, Alhazzani A, Yarascavitch B, Reddy K, Zaidat OO, Almenawer SA. Endovascular Thrombectomy for Acute Ischemic Stroke: A Meta-analysis. JAMA. 2015;314:1832-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 359] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 12. | Singh B, Parsaik AK, Prokop LJ, Mittal MK. Endovascular therapy for acute ischemic stroke: a systematic review and meta-analysis. Mayo Clin Proc. 2013;88:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E; SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 943] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 14. | Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, Cabrejo L, Slaoui T, Guidoux C, Lapergue B, Klein IF, Olivot JM, Abboud H, Simon O, Niclot P, Nifle C, Touboul PJ, Raphaeli G, Gohin C, Claeys ES, Amarenco P; RECANALISE investigators. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281-299. [PubMed] |
| 16. | Nijland R, van Wegen E, Verbunt J, van Wijk R, van Kordelaar J, Kwakkel G. A comparison of two validated tests for upper limb function after stroke: The Wolf Motor Function Test and the Action Research Arm Test. J Rehabil Med. 2010;42:694-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1707] [Cited by in RCA: 1285] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 18. | Arsava EM, Kim GM, Oliveira-Filho J, Gungor L, Noh HJ, Lordelo Mde J, Avery R, Maier IL, Ay H. Prediction of Early Recurrence After Acute Ischemic Stroke. JAMA Neurol. 2016;73:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;CD000197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 20. | Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Risk-benefit profile of long-term dual- versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann Intern Med. 2013;159:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3870] [Cited by in RCA: 3844] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 22. | McKay R. Stem cells in the central nervous system. Science. 1997;276:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1067] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 23. | Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752-4757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 306] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Gage FH. Mammalian neural stem cells. Science. 2000;287:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3484] [Cited by in RCA: 3413] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 26. | Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, Frisén J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 614] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 543] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 28. | Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón MA, Nakashima K, Colak D, Götz M, Fariñas I, Gage FH. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 29. | Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 954] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 30. | Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 31. | Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, Song H. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015;17:360-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 597] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 32. | Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1974] [Cited by in RCA: 1769] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 33. | Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Lim DA, Tramontin AD, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 790] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 35. | Kalladka D, Muir KW. Brain repair: cell therapy in stroke. Stem Cells Cloning. 2014;7:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 37. | Banerjee S, Williamson D, Habib N, Gordon M, Chataway J. Human stem cell therapy in ischaemic stroke: a review. Age Ageing. 2011;40:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 39. | Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, Miller K, Lincoln N, Partridge C, Wellwood I, Langhorne P. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke. 2004;35:2529-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 40. | Chen L, Zhang G, Gu Y, Guo X. Meta-Analysis and Systematic Review of Neural Stem Cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep. 2016;6:32291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Chollet F, Cramer SC, Stinear C, Kappelle LJ, Baron JC, Weiller C, Azouvi P, Hommel M, Sabatini U, Moulin T, Tardy J, Valenti M, Montgomery S, Adams H. Pharmacological therapies in post stroke recovery: recommendations for future clinical trials. J Neurol. 2014;261:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839-11844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 464] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 43. | Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 544] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 44. | Li J, Tang Y, Wang Y, Tang R, Jiang W, Yang GY, Gao WQ. Neurovascular recovery via co-transplanted neural and vascular progenitors leads to improved functional restoration after ischemic stroke in rats. Stem Cell Reports. 2014;3:101-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Andsberg G, Kokaia Z, Björklund A, Lindvall O, Martínez-Serrano A. Amelioration of ischaemia-induced neuronal death in the rat striatum by NGF-secreting neural stem cells. Eur J Neurosci. 1998;10:2026-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Chang DJ, Lee N, Choi C, Jeon I, Oh SH, Shin DA, Hwang TS, Lee HJ, Kim SU, Moon H, Hong KS, Kang KS, Song J. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transplant. 2013;22:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS One. 2007;2:e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Chen L, Zhang G, Khan AA, Guo X, Gu Y. Clinical Efficacy and Meta-Analysis of Stem Cell Therapies for Patients with Brain Ischemia. Stem Cells Int. 2016;2016:6129579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, Yoshikawa H, Stern DM, Matsuyama T, Taguchi A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Müller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 51. | Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 52. | Lee MC, Jin CY, Kim HS, Kim JH, Kim MK, Kim HI, Lee YJ, Son YJ, Kim YO, Woo YJ. Stem cell dynamics in an experimental model of stroke. Chonnam Med J. 2011;47:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke. 2012;7:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 55. | Jeong H, Yim HW, Cho YS, Kim YI, Jeong SN, Kim HB, Oh IH. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 2014;7:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18:59-64. [PubMed] |
| 57. | Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 58. | Marei HE, Hasan A, Rizzi R, Althani A, Afifi N, Cenciarelli C, Caceci T, Shuaib A. Potential of Stem Cell-Based Therapy for Ischemic Stroke. Front Neurol. 2018;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 59. | Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human Neural Stem Cell Therapy for Chronic Ischemic Stroke: Charting Progress from Laboratory to Patients. Stem Cells Dev. 2017;26:933-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Sakata H, Narasimhan P, Niizuma K, Maier CM, Wakai T, Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298-3310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Wu P, Tarasenko YI, Gu Y, Huang LY, Coggeshall RE, Yu Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Faiz M, Sachewsky N, Gascón S, Bang KW, Morshead CM, Nagy A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell. 2015;17:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 65. | Péron S, Berninger B. Imported Stem Cells Strike against Stroke. Cell Stem Cell. 2015;17:501-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, Götz M. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell. 2013;12:426-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 67. | Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3337] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 68. | Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010;88:3282-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Li X, Yang Z, Zhang A. The effect of neurotrophin-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2009;30:4978-4985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100:14421-14426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Zhang ZH, Wang RZ, Wang RZ, Li GL, Wei JJ, Li ZJ, Feng M, Kang J, Du WC, Ma WB, Li YN, Yang Y, Kong YG. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci Lett. 2008;444:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946-11950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1184] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 73. | Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 644] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 74. | Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, Zhu Y, Yang GY. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325-33; discussion 325-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, Tayra JT, Shinko A, Wakamori T, Agari T, Date I. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson's disease. Neurosci Res. 2011;71:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Xie Z, Han P, Cui Z, Wang B, Zhong Z, Sun Y, Yang G, Sun Q, Bian L. Pretreatment of Mouse Neural Stem Cells with Carbon Monoxide-Releasing Molecule-2 Interferes with NF-κB p65 Signaling and Suppresses Iron Overload-Induced Apoptosis. Cell Mol Neurobiol. 2016;36:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061-10065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 755] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 78. | Clark WM, Rinker LG, Lessov NS, Hazel K, Eckenstein F. Time course of IL-6 expression in experimental CNS ischemia. Neurol Res. 1999;21:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Wei L, Wei ZZ, Jiang MQ, Mohamad O, Yu SP. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol. 2017;157:49-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 81. | Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2014;141:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Rosenblum S, Smith TN, Wang N, Chua JY, Westbroek E, Wang K, Guzman R. BDNF Pretreatment of Human Embryonic-Derived Neural Stem Cells Improves Cell Survival and Functional Recovery After Transplantation in Hypoxic-Ischemic Stroke. Cell Transplant. 2015;24:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Boviatsis EJ, Kouyialis AT, Boutsikakis I, Korfias S, Sakas DE. Infected CNS infusion pumps. Is there a chance for treatment without removal? Acta Neurochir (Wien). 2004;146:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Harbaugh RE. Novel CNS-directed drug delivery systems in Alzheimer's disease and other neurological disorders. Neurobiol Aging. 1989;10:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081-5086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 86. | Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 87. | Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, Sinn DI, Lee YS, Kim SU, Kim M, Roh JK. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neurosci Res. 2005;53:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Zhang G, Guo X, Chen L, Li B, Gu B, Wang H, Wu G, Kong J, Chen W, Yu Y. Interferon-γ Promotes Neuronal Repair by Transplanted Neural Stem Cells in Ischemic Rats. Stem Cells Dev. 2018;27:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Xuan AG, Long DH, Gu HG, Yang DD, Hong LP, Leng SL. BDNF improves the effects of neural stem cells on the rat model of Alzheimer's disease with unilateral lesion of fimbria-fornix. Neurosci Lett. 2008;440:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Strecker JK, Olk J, Hoppen M, Gess B, Diederich K, Schmidt A, Schäbitz WR, Schilling M, Minnerup J. Combining Growth Factor and Bone Marrow Cell Therapy Induces Bleeding and Alters Immune Response After Stroke in Mice. Stroke. 2016;47:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Broughton BR, Lim R, Arumugam TV, Drummond GR, Wallace EM, Sobey CG. Post-stroke inflammation and the potential efficacy of novel stem cell therapies: focus on amnion epithelial cells. Front Cell Neurosci. 2013;6:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 569] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 93. | Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 541] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 94. | Newman MB, Misiuta I, Willing AE, Zigova T, Karl RC, Borlongan CV, Sanberg PR. Tumorigenicity issues of embryonic carcinoma-derived stem cells: relevance to surgical trials using NT2 and hNT neural cells. Stem Cells Dev. 2005;14:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1011] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 96. | Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 649] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 97. | Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 98. | Menendez S, Camus S, Herreria A, Paramonov I, Morera LB, Collado M, Pekarik V, Maceda I, Edel M, Consiglio A, Sanchez A, Li H, Serrano M, Belmonte JC. Increased dosage of tumor suppressors limits the tumorigenicity of iPS cells without affecting their pluripotency. Aging Cell. 2012;11:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 99. | Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86:1936-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 550] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 102. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6160] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 103. | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4254] [Cited by in RCA: 4123] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 104. | Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mäger I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120:1632-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 755] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 105. | Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 106. | György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 107. | Malhotra H, Sheokand N, Kumar S, Chauhan AS, Kumar M, Jakhar P, Boradia VM, Raje CI, Raje M. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J Biomed Nanotechnol. 2016;12:1101-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 108. | Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3959] [Cited by in RCA: 4132] [Article Influence: 413.2] [Reference Citation Analysis (0)] |
| 109. | Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 2585] [Article Influence: 287.2] [Reference Citation Analysis (0)] |
| 110. | Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab AS, Baban B, Dhandapani KM, Hess DC, Hoda MN, Stice SL. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl Stroke Res. 2018;9:530-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 111. | Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke. 2018;49:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 112. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2580] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 113. | Marbán E. The Secret Life of Exosomes: What Bees Can Teach Us About Next-Generation Therapeutics. J Am Coll Cardiol. 2018;71:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 114. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1230] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 115. | Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 116. | Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 117. | Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest. 2016;126:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |