Published online Sep 26, 2018. doi: 10.4252/wjsc.v10.i9.119
Peer-review started: April 27, 2018
First decision: June 6, 2018
Revised: July 27, 2018
Accepted: August 5, 2018
Article in press: August 5, 2018
Published online: September 26, 2018
Processing time: 152 Days and 1 Hours
Balanced sphingolipid signaling is important for the maintenance of homeostasis. Sphingolipids were demonstrated to function as structural components, second messengers, and regulators of cell growth and survival in normal and disease-affected tissues. Particularly, sphingosine kinase 1 (SphK1) and its product sphingosine-1-phosphate (S1P) operate as mediators and facilitators of proliferation-linked signaling. Unlimited proliferation (self-renewal) within the regulated environment is a hallmark of progenitor/stem cells that was recently associated with the S1P signaling network in vasculature, nervous, muscular, and immune systems. S1P was shown to regulate progenitor-related characteristics in normal and cancer stem cells (CSCs) via G-protein coupled receptors S1Pn (n = 1 to 5). The SphK/S1P axis is crucially involved in the regulation of embryonic development of vasculature and the nervous system, hematopoietic stem cell migration, regeneration of skeletal muscle, and development of multiple sclerosis. The ratio of the S1P receptor expression, localization, and specific S1P receptor-activated downstream effectors influenced the rate of self-renewal and should be further explored as regeneration-related targets. Considering malignant transformation, it is essential to control the level of self-renewal capacity. Proliferation of the progenitor cell should be synchronized with differentiation to provide healthy lifelong function of blood, immune systems, and replacement of damaged or dead cells. The differentiation-related role of SphK/S1P remains poorly assessed. A few pioneering investigations explored pharmacological tools that target sphingolipid signaling and can potentially confine and direct self-renewal towards normal differentiation. Further investigation is required to test the role of the SphK/S1P axis in regulation of self-renewal and differentiation.
Core tip: The aim of this study is to review the role of sphingosine kinase, sphingosine-1-phosphate (S1P), and its receptors in the regulation of stem/progenitor cell function. Our analysis indicates that S1P receptor expression, localization, and specific downstream effectors influence the rate of self-renewal and differentiation of myogenic, hematopoietic, endothelial, neural, and cancer progenitor cells.
- Citation: Ng ML, Yarla NS, Menschikowski M, Sukocheva OA. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J Stem Cells 2018; 10(9): 119-133
- URL: https://www.wjgnet.com/1948-0210/full/v10/i9/119.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i9.119
During organism growth, development, and adaptation to changed environmental conditions, orchestrated functioning of multiple processes supports physiological homeostasis. The synchronization should occur at the level of a single cell, such as controlled cell division and apoptosis, and at the level of organs and systems, such as directed angiogenesis, immune responses, and regeneration. Many of those biological processes have been shown to rely on the sphingolipid signalling cascade. An important member of the sphingolipid family, sphingosine-1-phosphate (S1P), is a bioactive signalling molecule. S1P effects are essential for structural and functional regulation of cell growth and survival. The main source of S1P is catabolic degradation of membrane glycosphingolipids and sphingomyelin, which results in production of sphingosine which, in turn, is phosphorylated by sphingosine kinases (SphK1 and SphK2)[1,2]. Supported by experimental evidence observed in Sphk knockout mice in vivo, SphK isozymes can partially balance metabolism for each other, although there are some SphK1- and SphK2-specific non-overlapping functions[3,4].
Suggestively, S1P is generated during membrane restructuring in all types of cells. Locally produced S1P can act either intracellularly or extracellularly. S1P can be released to the extracellular environment by erythrocytes[5], platelets[6], and endothelial cells[7]. Circulating S1P is an important signalling mediator and ligand for specific G protein-coupled receptors S1Pn (n = 1 to 5)[8,9]. S1P1 is highly expressed in various tissues, but specifically in endothelial cells and vasculature. S1P2 and S1P3 are also broadly expressed, although their levels of expression demonstrated some function specificity. Activated S1P receptors trigger distinctive downstream effectors and respective responses[10,11]. Intracellularly produced S1P can be utilized in two different metabolic pathways[8,12]. Firstly, S1P can be recycled through ceramide synthesis by S1P-specific phosphatases[13]. Secondly, S1P can be irreversibly degraded by S1P lyase into phospho-ethanolamine and hexadecenal linked to a variety of intracellular signalling cascades[14].
Various growth stimulating agents, hormones, and cytokines, the canonical regulators of cell proliferation and survival, can activate SphK and stimulate S1P production. Hormones, cytokines, and growth factors including EGF[15], PDGF[16], IGF[17], VEGF[18], NGF[19], TGF[20], TNF[21], and the steroid hormone estrogen[15,22,23] were shown to trigger SphK1/S1P signaling in different cells. Supporting the global role of the sphingolipid network in regulation of proliferation, the list of SphK/S1P-inducing agents keeps growing. Recent experimental findings demonstrate that S1P and its network play a complex role in the regulation of stem/progenitor cell signalling in normal and malignant tissues.
Stem or progenitor cells are defined as undifferentiated cells with specific clonogenic potential, unlimited self-renewal capacity that is accompanied by directed differentiation into multiple (often limited to a specific number) cell lineages[24,25]. According to their programmed differentiation potential, stem cells are encoded for particular tissue regeneration and cell replacement. For instance, pluripotent embryonic stem cells (ESCs) can differentiate into cell-types of all the primary germ layers. Bone marrow (BM)-located adult stem cells are considered multipotent[26] or pluripotent[27,28]. Other groups of adult stem cells are oligopotent, bipotent, or unipotent and are represented by basal cells in the epidermis, spermatogonial stem cells, and satellite cells in skeletal muscles[28]. The cells with limited potency are often referred to as progenitor cells and include, for instance, endothelial progenitor cells (EPCs)[29] and pancreatic progenitor cells[30]. Progenitor cells are marked not only by limited number of divisions, but also higher levels of directed lineage differentiation.
The core properties of ESC pluripotency are maintained by a group of lineage-specific transcription factors (TFs) such as Nanog, Oct4, and Sox2-NOS and their regulatory networks[31]. Recently, it was demonstrated that high intracellular levels of S1P is associated with increased mouse ESC proliferation and higher expression of the cell surface pluripotency markers SSEA1 and Oct4[31]. The authors found that ESCs express high level of sphingosine phosphate lyase (SPL), an enzyme that catalyzes the S1P degradation, thus, keeping the intracellular level of S1P under tight control[32]. During the last decade, besides the detected effects in ESCs, the regulatory role of sphingolipids has been assessed in several types of precursor multipotent cells including neural, muscle, hematopoietic, endothelial, and mesenchymal progenitor/stem cells. S1P was suggested to functions as a trophic factor for many stem cell types. However, the role of sphingolipids in the regulation of cell renewal and differentiation remains only partially addressed. Here, we review and discuss recent advancement and development about the functional role of sphingosine kinase, S1P and S1P receptors in stem/progenitor cells.
Hematopoietic stem cells (HSCs) represent the rare population of precursor cells that defines the blood composition and homeostasis. HSCs are characterized by their unique capacity for self-renewal and multi-lineage differentiation. HSCs and downstream partially lineage-committed progenitor cell functions are tightly linked to their migratory properties, especially during fetal development[33,34]. Although the majority of postnatal and adult HSCs/progenitors stay in BM specialized niches or cavities[35], some of the HSCs/progenitors belong to a highly migratory subpopulation that recirculates between BM and blood[36,37]. Suggestively, the HSC/progenitor trafficking mechanism supports full occupancy of stem cell niches in all BM cavities[37,38]. HSC trafficking is directed by an S1P blood/lymph/tissue gradient that is mostly maintained by SphK/S1P receptor and S1P-degrading enzyme S1P lyase network[38]. Notably, another well-studied sphingolipid, ceramide-1-phosphate (C1P), can also enhance the migration of endothelial and lymphoid progenitor cells[39], suggesting that other sphingolipid family members should be tested for potential involvement in the regulation of hematopoietic stem/progenitor cell functions.
S1P concentration in peripheral blood and lymph regulates HSC and lymphocyte egress from lymphoid organs[34,37]. HSCs and progenitors express S1P1 receptor(s) that can sense blood plasma S1P and direct stem cell migration[40]. Although water-insoluble S1P binds apolipoprotein M and circulates in peripheral blood mostly as a part of high-density lipoprotein (HDL) particles, the level of S1P is always higher in plasma and lymph compared to the S1P level in interstitial fluids of all organs, including thymus and lymph nodes[41,42]. The concentration gradient serves as a chemoattractant to direct the migration of S1P1-expressing cells from BM to peripheral blood[37,40]. Similarly, lymphocyte egress from lymph nodes is directed by the S1P gradient between lymphoid tissue and lymph[38]. The level of S1P1 receptor expression is a critical factor that regulates sensitivity to circulating S1P. Expressed in blood or lymph-circulating cell, S1P1 receptors are rapidly internalized and downregulated through G-protein coupled receptor kinase-2–mediated phosphorylation[43]. Inside of tissue, S1P1 is up-regulated under the condition of low-S1P concentration in interstitial fluids. The high level of S1P1 on tissue-located cells supports the traversing of cells from tissues towards high S1P in blood plasma or lymphatics. Animal HSCs also express S1P1 receptors that mediate stem cell trafficking from BM into peripheral blood[44]. The mouse model has allowed for the use of the specific S1P1 inhibitor W146, which confirmed a key role for the receptor in BM retention of hematopoietic cells[44].
Three different S1P receptors, including S1P1, S1P2, and S1P3, influence the development and function of the embryonic vasculature[45,46]. The S1P/S1P1 axis plays a leading role during embryonic vascularization and angiogenesis. Supporting a functional link between the endothelial and red blood cell network, S1P synthesis and release from erythrocytes is required for embryonic vascular development[47]. S1P2 and S1P3 effects are considered important, although as accessory or partially redundant in some studies[11,12].
Activated S1P1 receptor has been found to stimulate the proliferation of endothelial vascular (outgrowth) progenitors[48] and colony-forming cells[49]. Morphogenesis of the kidney vasculature is also mediated by S1P1 signalling. A hypothetical endothelial and hematopoietic precursor has been shown to express S1P1 receptor in the kidney. The receptor activation maintains an appropriate development of glomerular capillaries, arterial mural cell coating, and lymphatic vessel development[50]. Besides S1P1, S1P3 receptor positively directs capillary-like formation and EPC migration. Notably, S1P2 partially blocks the migratory capacity of mesenchymal progenitor cells, mesangioblasts, whereas SphK and/or S1P1/S1P3 are involved in the positive regulation of angiogenesis in vivo[20,45]. S1P2 signalling is clearly tissue-specific and can promote proliferation in different cells similarly to S1P1 and/or S1P3. Accordingly, small hepatocyte-progenitor and stem (oval) cell proliferation is positively associated with S1P2 and S1P4 expression during liver injury[51]. Furthermore, S1P2 promotes the growth of primary CD34+ mononuclear cells obtained from chronic myeloid leukemia (CML) patients[52].
The S1P-producing enzyme SphK1 is partially responsible for the maintenance of the EPC-specific phenotype. SphK1 controls the rate and direction of EPC differentiation, although the enzyme expression level did not affect the hematopoietic compartment[53]. The authors detected high levels of SphK1 activity in EPCs, which gradually decreased in more differentiated endothelial cells. Notably, SphK1 knockout mice demonstrated higher levels of circulating EPCs[53]. The data suggests a potential negative role of the enzyme in the regulation of vascular regeneration when the presence of EPCs in circulation is highly desirable, although the question requires further investigation. Overexpression of SphK1 facilitates the retention of EPCs at the progenitor stage with probable delay in the following differentiation program, but that was not tested. Suggestively, SphK1 function in EPCs can be replaced by SphK2. The role of SphK2 in the regulation of EPC function remains unclear.
The S1P3 receptor axis influences some specific S1P responses in EPCs. Patient-derived EPCs were tested for the activation of G protein-coupled protein receptor C-X-C chemokine receptor 4 (CXCR4) signalling. CXCR4 axis is an important regulator of pluripotent cell development and function, as it is involved in the interaction between HSCs cells or hematologic and solid tumor cells and their protective microenvironment[54]. It was detected that the S1P/S1P3 axis positively induced the CXCR4-dependent signalling pathway[55]. Furthermore, the specific S1P1 receptor antagonist FTY720 increased CXCR4-dependent chemotactic responsiveness and migration of human CD34+ lineage-committed progenitor cells[56]. Similarly, besides EPCs, S1P1 and S1P3 activation was required in the regulation of CSCs migration[57,58].
S1P1 effects were tested in megakaryocytes, the thrombocyte lineage-specific progenitors. S1P1 is involved in the initiation of the elongation of trans-endothelial pro-thrombocyte extensions into BM sinusoids and activates the subsequent shedding of thrombocytes[59]. During activation, platelets can release considerable amounts of S1P and further increase S1P concentration in blood plasma besides the release of the lipid from erythrocytes and endothelial cells[60]. The sudden local increase in S1P concentration is potentially associated with activation of immune cell migration. The role of platelet-derived S1P in the regulation of HSCs and/or progenitor trafficking requires further testing.
Skeletal muscles are formed by myoblasts, muscle cell progenitors. The multistage process of myogenesis is preceded by myoblast division, which is followed by terminal differentiation, cell merging into multinucleated myofibers, and maturation[61-63]. The initiation of the myoblast differentiation process is marked by progenitor cell cycle cessation accompanied by vigorous synthesis of myogenin and expression of muscle-specific proteins, including sarcomeric components and creatine kinase[61]. Represented by quiescent mononucleated satellite cells, adult muscle cell progenitors employ a similar differentiation program as developing myoblasts[62]. The satellite cells/myoblasts, although mitotically quiescent, can be induced to proliferate by physical trauma, weight bearing, or inflammation-induced trauma[64]. After multiple rounds of satellite cell divisions, the cell cycle stops and the newly produced cells fuse onto the existing damaged muscle fibers. This process was observed in vitro using C2C12 cells, a skeletal myoblast cell line derived from murine satellite cells[65-67]. Despite significant progress, the molecular mechanisms of myogenesis remain only partially explored. For instance, molecular regulation of muscle progenitor signalling and associated repair mechanisms remain largely unclear, although growth factors and cytokines have been confirmed to regulate skeletal muscle biology[63]. Sphingolipids can transduce signalling from growth factor and cytokine receptors as messengers and amplifiers in a large variety of cells[11]. Consequently, the role of sphingolipids has been examined in the regulation of myogenesis.
Traumatic tissue injury and subsequent inflammatory activation of leukocytes and macrophages are marked by the release of cytokines and growth factors that can stimulate skeletal muscle regeneration and remodelling[63,68]. One of the keystone recent discoveries demonstrated a direct link between sphingolipid signalling and trauma/inflammation-provoked responses in muscle progenitor cells[67]. Bradykinin and its related peptides are pro-inflammatory molecules and muscle-specific growth factors that mediate exudative and inflammatory phases of muscle healing[69,70]. Bradykinin is also the leading member of the kinin/kallikrein system, which directs inflammation-linked responses in mesenchymal cells including fibroblasts, myofibroblasts, and smooth muscle cells[70]. Bradykinin has been shown to induce myogenic differentiation in C2C12 myoblasts through SphK1, the specific S1P-transporter spinster homolog 2 (Spns2), and S1P2 receptor. Specific pharmacological inhibition and/or protein expression silencing was used to confirm the involvement of the S1P axis in bradykinin-induced myogenic differentiation[67].
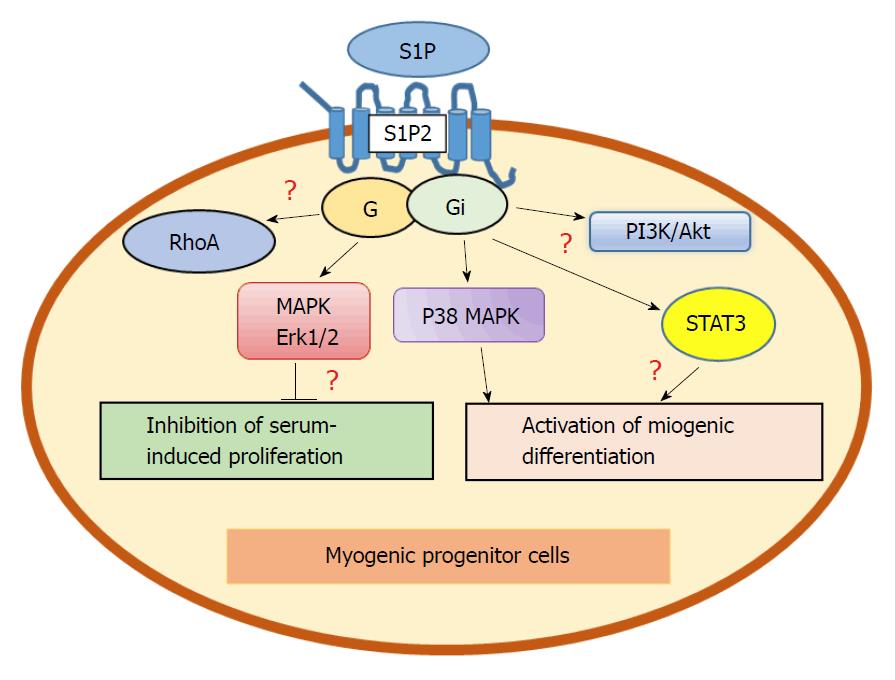
Serving as a muscle trophic factor, S1P has been suggested to play a leading role in the stimulation of myogenesis and regeneration provoked by various agents via transactivation of the S1P2 receptor pathway[65,66,71,72]. Extracellular S1P reduced serum-induced cell proliferation, promoted cell cycle exit, and up-regulated the expression of various differentiation markers in myoblasts. The S1P-dependent myogenic differentiation is mediated by S1P2, activation of ERK1/ERK2 and p38 MAPK, both identified as downstream effectors of S1P2[71]. Furthermore, insulin growth factor 1 (IGF-1) increased SphK activity and induced transactivation of the S1P2 receptor in C2C12 murine myoblasts. The activation was linked to the IGF-1 myogenic differentiation effect. Pharmacological inhibition of SphK, specific silencing of SphK1 or SphK2, and S1P2 receptor downregulation resulted in reduction of the IGF-1 pro-differentiating effect in myoblasts[66]. Interestingly, IGF-1 also activated S1P1/S1P3 receptors. Contrary to S1P2, S1P1/S1P3 negatively regulated the IGF-1-induced mitogenic differentiation. Specific silencing of S1P1/S1P3 receptors notably stimulated myoblast proliferation[66]. The data correspond to the growth-stimulating signalling of S1P3 in tumors where the ability of sphingolipids to mediate IGF-1 effects is well recognized[11]. The myogenesis-stimulating role of S1P2 is partially unexpected, as S1P2 anti-proliferative effects were previously observed in various, although mostly not stem-like cells[11,12]. S1P2 was shown to inhibit Rac signalling and related cell migration contrary to its demonstrated effects in myoblasts[73]. However, the divergence might be associated with the high specialization of stem cells and adjustments to pluripotency of the S1P2 signalling network. Notably, S1P2 induced the myogenic differentiation program independently of acute S1P treatment[67]. Conclusively, the pleiotropic role of SphK/S1P receptor axis in skeletal myoblasts reflects the association of S1P receptor expression pattern with contrasting biological responses.
Proliferative and chemotactic effects of vascular endothelial growth factor (VEGF) signalling are also transduced by SphK/S1P network in muscle progenitor cells[74]. Previously, VEGF signalling pathway was shown to interact with the SphK/S1P axis in several types of normal[75] and malignant cells[12,18]. SphK activation and S1P1 expression can be induced by VEGF. S1P1 and VEGF receptor-2 (VEGFR-2) proteins were found to interact and form a signalling complex[11,18]. The interaction was described as mutual, as S1P enhanced the levels of VEGF expression and transactivated VEGFR-2[11,18,75]. The role of S1P in the mediation of VEGF myogenesis-related effects was confirmed in another study that tested the role of bone-marrow-derived mesenchymal stromal cells (MSCs) as regulators of myogenesis. MSCs synthesize and release a large amount of S1P, which assists in skeletal muscle healing[74]. Conditioned media with MSC-secreted S1P stimulated C2C12 myoblast and satellite cell proliferation. A similar effect was reached by exposure to VEGF, as the myoblast growth response to MSC-secreted VEGF also induced S1P release from C2C12 cells[74,75].
Notably, the involvement of S1P2 and S1P3 receptors in the regulation of myogenesis was detected more than a decade ago[76]. Meacci et al[76] observed that myogenic differentiation was accompanied by a significant variation in S1P receptor expression levels. The authors also suggested that the S1P signalling axis is a key component required for sphingolipid effects in proliferating muscle cells[76]. However, the authors observed that the S1P2 receptor is down-regulated during myogenesis, while SphK was enhanced in differentiating C2C12 myoblasts[77]. Suggestively, S1P2 and S1P3 can be activated during different stages of myogenesis and stimulate alternative biological responses in regular and progenitor muscle cells. For instance, S1P3 levels are high in quiescent murine myogenic cells, but decrease during cell cycle initiation[78]. Constitutive expression of S1P3 resulted in the suppression of satellite cell cycle progression. S1P3-null satellite cells exhibited enhanced proliferation. Acute cardiotoxin-induced muscle regeneration was promoted in S1P3-null myoblasts in vivo, marked by bigger muscle fibers compared to control mice. The data are supported by experimental observations in the mdx mouse model of Duchenne muscular dystrophy. S1P3 knockdown produced a less severe muscle dystrophic phenotype, indicating that the S1P3-linked pathway represses cell cycle progression to direct myoblast functions[77].
Myoblasts and fully differentiated muscle cells are marked by a heterogeneous expression pattern of S1P receptor subtypes. S1P1 has been mostly found in cardiomyocytes, while S1P2/S1P3 receptors are expressed by cardiac progenitor cells[78]. S1P receptor activates Rho signalling, which in turn, switches in the proliferation of cardiac myoblasts. Notably, both S1P2 and S1P3 induce RhoA activation through Gα12/13 during myocardial regeneration, indicating some redundancy of signalling pathways[78]. However, there is some specificity for different S1P receptor subtypes. For instance, during construction of the primary heart tube in zebrafish, S1P2 controls proper endoderm formation and cardiac myoblast migration[79]. Notably, in another study, S1P2 negatively regulated satellite cell migration, while S1P1/S1P4 facilitated the S1P migratory effect in myoblast cells[72].
Considering muscle-specific cytoskeletal remodeling, the role of specific S1P receptors is unclear. Myoblasts and satellite cell differentiation capacity depends on cytoskeletal remodelling and can be controlled by gap junction proteins, particularly connexin (Cx) 43[80]. It has been shown that S1P induces p38 MAPK activation, phosphorylation of Cx43 and association of Cx43 with cortactin and F-actin, followed by murine C2C12 myoblasts differentiation[81]. S1P-induced C2C12 myoblast differentiation and transient receptor potential canonical 1 (TRPC1) channel activity have been linked to Cx43 expression/function via calpain/PKCα axis[76], although the involvement of S1P receptor has not been demonstrated.
Transforming growth factor beta 1 (TGFβ1), inflammation-associated pleiotropic cytokine, was shown to control skeletal muscle regeneration via S1P3 receptor signalling[65]. TGFβ1 increased levels of SphK1 in C2C12 myoblasts in a Smad-dependent manner and stimulated the expression of S1P3 receptors that resulted in induction of fibrosis. The study demonstrated the involvement of Rho/Rho kinase signalling downstream of S1P as profibrotic TGFβ1 effect[65]. Notably, S1P receptors are linked to various downstream effectors in myoblasts. For instance, S1P2 myogenic signalling is mediated by activated phosphatidylinositol 3-kinase (PI3K)[72] and signal transducer and activator of transcription 3 (STAT3)-dependent pathways[82]. The biological meaning of the divergence of S1P receptor signalling requires further clarification (Figure 1). For instance, Rho signalling that can mediate S1P effects in non-pluripotent cells[73] is also activated in cardiac myoblasts[76], suggesting that S1P isoforms might be linked to similar downstream effectors independently of pluripotency. Future studies should clarify how S1P receptors induce different effects in normal, malignant, and progenitor cells using similar downstream effectors.
It has been shown that myogenesis is regulated not only by SphK/S1P receptors, but also by other S1P metabolizing enzymes, including S1P lyase. The lyase irreversibly catabolizes S1P at carbon bond C(2-3), producing hexadecenal and ethanolamine-phosphate. The lyase enhances apoptosis induced by chemotherapy, radiation and ischemia in different cells[82]. Undetectable in resting skeletal muscle, the S1P lyase level is upregulated after muscle injury[82]. The mdx mouse model for muscular dystrophy was marked by skeletal muscle S1P lyase upregulation and S1P deficiency in vivo. Accordingly, pharmacological S1P lyase inhibition stimulated an increase in muscle S1P levels and myoblast recruitment, thus advancing mdx skeletal muscle regeneration[82]. S1P lyase knockdown cells demonstrated increased levels of intra- and extra-cellular S1P, but decreased myotube formation and delayed induction of three myogenic microRNAs (miRNAs) including miR-1, miR-206, and miR-486[83]. Myotube formation was recovered in cells treated with an S1P1 agonist, S1P2 antagonist, and combination treatments. Transfection with miR-1 or miR-206 allowed the S1P lyase knockdown cells to reverse the inhibition of differentiation[83]. Considering that stem cell resistance to apoptosis is a keystone of regeneration, pharmacological inhibition of S1P lyase during specific stages of myogenesis seems like an attractive therapeutic approach to enhance muscular remodelling after injury[78,82].
Another sphingolipid C1P has been implicated in the regulation of skeletal muscle regeneration. C1P induced myoblast proliferation and myoblast cell cycle progression without activation of a putative G(i)-coupled C1P receptor[84]. C1P stimulated the phosphorylation of glycogen synthase kinase-3β and the production of retinoblastoma gene, and enhanced cyclin D1 protein levels. Furthermore, various downstream target proteins, including phosphatidylinositol 3-kinase/Akt, ERK1/2, and the mammalian target of rapamycin, mediated C1P signalling in myoblasts. Interestingly, C1P did not influence the induction of myoblast apoptosis or myogenic differentiation[84]. Previous knowledge of C1P signalling is limited to the demonstrated effects in fibroblasts and macrophages, thus, demanding further investigation of C1P role in progenitor cells.
According to the recently developed theory, brown adipose cells are derived from a mesenchymal progenitor that shares some similarity with muscle cell precursor cells and expresses Myf5-Cre proteins, while white adipocytes originate from a Myf5-negative precursor[85]. According to another theory, adipocytes arise from a vascular bed and originate from a subset of endothelial cells[86]. While the theory is clarified, S1P was revealed to promote differentiation of C3H10T1/2 multipotent mesenchymal stem cells into osteogenic rather than adipogenic progenitors[87]. Furthermore, adipose tissue itself was shown to contain stem cell progenitors. The adipose stromal-vascular cell fraction is an abundant source of both multipotent and pluripotent progenitor cells, defined as adipose-derived stem cells. S1P1 is involved in the induction of adipose-derived stem cell growth by HDL[88].
Obesity and metabolic disorders might be associated with dysfunctional adipose progenitor cells and diabetes. Notably, multipotent pancreatic progenitor cells (MPCs) have been suggested as a promising target for the treatment of type-1 diabetes mellitus[30]. MPCs are stem cells with limited potency that proliferate and differentiate into three distinct lineages, including insulin-producing β cells, acinar cells, and ductal cells. The early MPCs were classified by the expression of the TFs Pdx1, Ptf1a, and Sox9, some of them known as mesenchymal progenitor markers[89]. High levels of Notch and Hippo/YES signalling are also required to maintain tree-like branched epithelium and block early MPC differentiation[90,91]. The SphK/S1P/S1P2 axis was found to support pancreatic progenitor differentiation[92]. S1P2, SphK1, and SphK2 expression levels were upregulated during pancreas second transition in the developing epithelium and co-localized with both trunk and tip progenitors[92]. S1P2 receptor activated YES-associated protein (YAP) and up-regulated connective tissue growth factor signalling important for the survival of endocrine and acinar pancreatic progenitors. S1P signalling decreased Notch regulation of lineage allocation necessary for endocrine and acinar specification[92]. S1P2 receptor-null embryos demonstrated high perinatal mortality marked by pathological hematopoietic and vascular system phenotypes[46]. Expression of a negative posttranscriptional regulator of Notch signalling protein Sel1l was also influenced by S1P2 signalling. S1pr2 inhibition resulted in the loss of the Sel1l protein, which in turn is required to maintain normal Notch signalling and proper acinar and endocrine differentiation[92]. Given such an important role of S1P2 in the regulation of MPC differentiation, the role of S1R receptors in the regulation of differentiation should be further explored in future studies.
Conclusively, the sphingolipid signalling network is a potential therapeutic target to influence myogenesis, adipogenesis, and associated metabolic pathologies including diabetes. Pharmacological control over sphingolipid signalling should be tested during induction of muscle regeneration, aging, inflammation and trauma-associated muscle fibrosis. The role of the SphK/S1P axis in the regulation of adipose cell precursor function and adipose-derived stem cell differentiation remains to be clarified in future studies.
S1P signalling exhibited neuro-protective effects as a mediator of nerve growth factor (NGF) in hippocampal neurons and pheochromocytoma PC12 cells[93,94]. During the last couple of decades, several groups have addressed the role of sphingolipids in neural progenitor cells. Neural progenitor/stem cells (NPSCs) have limited potency, though they are still promising to for the treatment of Alzheimer’s disease[95] and brain or spinal cord injuries[96]. Besides insufficient proliferation rates, NPSCs maintain slow differentiation and migration characteristics. However, similar to its effects on circulating immune cells in blood and lymph, extracellular S1P is a powerful chemoattractant for microglial cells in the brain. More effective than fibroblast growth factor (FGF), S1P is a powerful stimulator of neurogenesis[97] (Figure 2). S1P can mediate FGF signalling in different neural cells. It was found that FGF coordinates S1P release from astrocytes. The extracellular S1P, through autocrine or paracrine mechanisms, stimulates astrocyte differentiation mediated by S1P receptors[98]. Previously, it was demonstrated that cerebellar astrocytes express S1P1, S1P2, and S1P3 receptors[99]. Another study indicated that S1P3 is overexpressed in astrocytes under pro-inflammatory conditions[100]. However, astrocytes are not true progenitor cells, but rather precursor cells. Furthermore, S1P receptor demonstrates heterogeneous expression in neural and NPSCs[101,102]. For instance, up-regulation of S1P1 was noted in NPSCs that migrate out of the embryoid body/ESCs[103]. However, the level and role of S1P receptor subtypes in NPSCs remains controversial, as different studies have demonstrated noticeable variations in S1P receptor expression[104].
In the presence of activated astrocytes, S1P further enhances NPSCs differentiation, indicated by neurite outgrowth and arborization[97,105]. Notably, neural precursors derived from ESCs express all five S1P receptor mRNAs, although S1P2 and S1P3 mRNA levels are the highest[97,105]. The effect of S1P on NPSCs is mediated by increased laminin expression and extracellular matrix (ECM) interactions with progenitor integrins. However, the role of particular S1P receptor subtypes has not been verified. For instance, the role of S1P2 remains unclear. Kimura et al[106] demonstrated that NPSC migration to sites of injury was inhibited by S1P2 activation.
The role of SphK/S1P in neural stem cells has been explored by Meng et al[107]. The authors detected SphK1 expression in neuron and progenitor cells of nascent trigeminal and dorsal root ganglia of the mouse embryo. The enzyme was found to increase NPSC proliferation and survival during early sensory ganglia development[107]. Embryos with both Sphk1 and Sphk2 genes knocked out displayed clear developmental defects marked by fewer neurons and progenitor cells in trigeminal and dorsal root ganglia[107]. This finding supports the previously shown data of crucial involvement of SphK1/S1P axis in the regulation of cell growth and survival in the developing neural system[107-110]. According to the proposed mechanism, sphingolipids are involved in neural cell signalling downstream of p75 and/or neurotrophin receptor pathways[107,111-117].
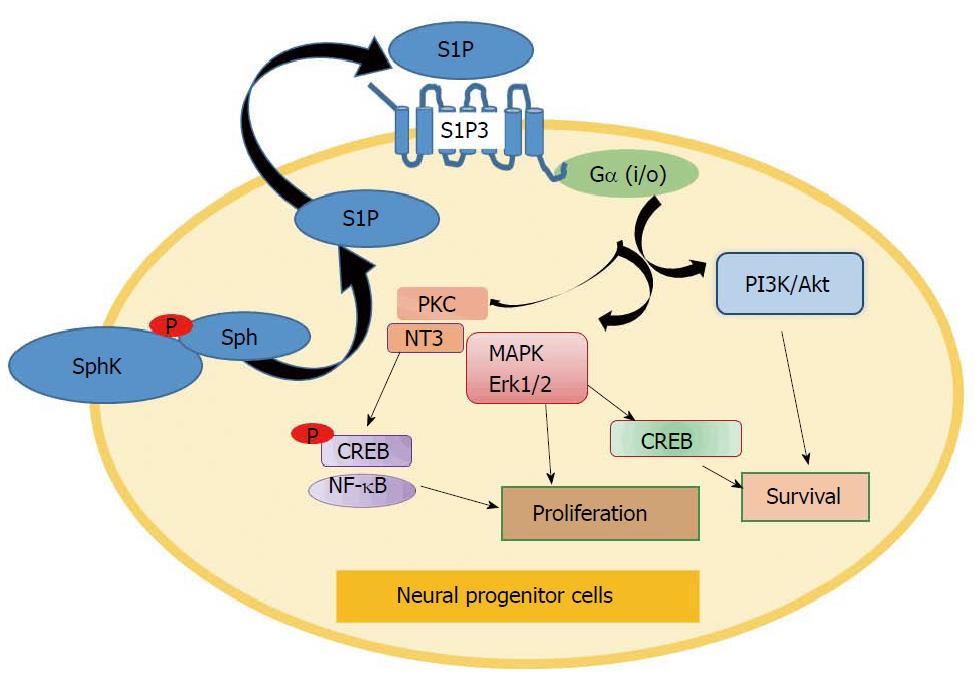
To establish a role for S1P in the regulation of neural cell survival, Saini et al[118] tested the involvement of SphK1 in the neurotrophin-3 (NT-3) signalling pathway. It was found that SphK1 mediates NT-3–dependent activation of cAMP-response element binding protein (CREB) in cultured oligodendrocyte progenitors. NT-3 increased SphK1 activation and translocation from the cytoplasm to the plasma membrane of oligodendrocytes. The effects coincided with enhanced S1P accumulation at the membrane. Downregulation of SphK1 facilitated apoptosis in oligodendrocyte progenitors induced by growth factor deprivation. Inhibition of Erk1/2 and PKC also blocked NT3- and S1P-induced CREB phosphorylation, indicating a concerted interaction among NT-3, SphK, Erk1/2 and PKC pathways[115,119]. Crosstalk between NT-3 and SphK1 has been also demonstrated in animal models of multiple sclerosis[120]. Notably, PTEN and Notch signalling mediate the anti-fibrotic effects of dihydro-S1P in systemic sclerosis[121]. However, a complexity of functional crosstalk between NT-3 and SphK signalling requires further clarification during oligodendrocyte development.
Survival-related mechanisms of S1P effects were linked to multiple signalling pathways. For instance, S1P can activate membrane S1P receptor(s) to induce CREB phosphorylation in oligodendroglial progenitors[122-124]. Downstream S1P receptor effects were mediated by activation of Erk1/2 and PKC-dependent pathways in progenitor cells[102,122]. Another signalling mechanism was associated with activation of growth factor signalling[93]. For instance, platelet-derived growth factor (PDGF) receptor was shown to activate SphK1 in oligodendrocytes[123]. In turn, SphK1 mediated PDGF-dependent up-regulation of mRNAs encoding the Kv1.5 and Kv1.6 K+ channels during oligodendrocyte proliferation[124]. Furthermore, SphK1 promoted survival of oligodendrocyte progenitors via upregulation of the anti-apoptotic protein Bcl-2 and downregulation of the pro-apoptotic protein Bim in a CREB-dependent manner. The mechanism is based on the established SphK1-dependent regulation of a balance between pro-apoptotic and anti-apoptotic Bcl-2 proteins shown in normal and cancer cells[11]. Considering genomic and epigenetic regulation, SphK1 can trigger the activation of various TFs, including AP-1 and NF-κB, which were shown to promote anti-apoptotic signalling[125-129]. Involvement of SphK/S1P/S1P3 receptor-dependent signalling in the regulation of survival and differentiation of neural stem/progenitor cells is summarized in Figure 2.
S1P receptor demonstrates heterogeneous expression in neural cells. Brain white matter cells contain the highest expression of S1P2[130]. S1P5 is expressed by mature oligodendrocytes where the receptor regulates survival and cell process retraction[131]. Higher expression level and activation of S1P1 facilitates survival of oligodendrocyte progenitors and induces oligodendroglial differentiation[132]. NPSCs derived from ESCs express all five S1P receptor mRNAs, although the actual protein expression level was not tested[105].
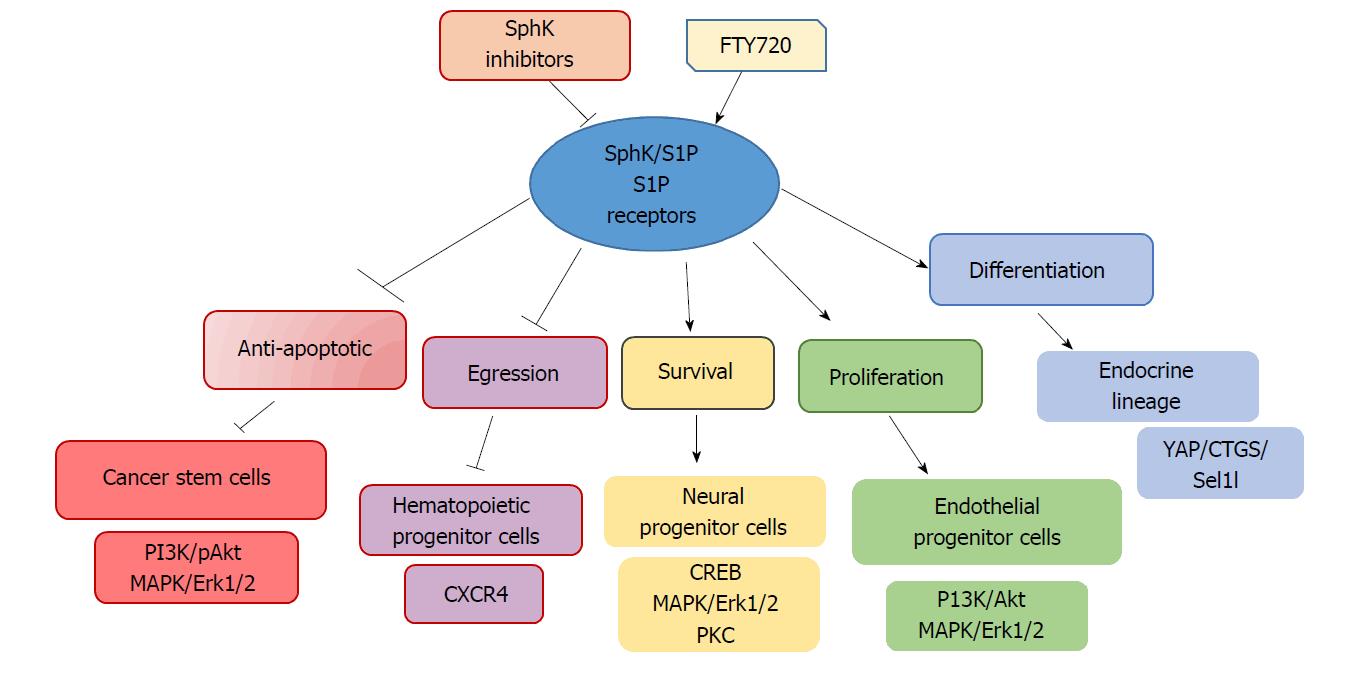
The level of S1P receptor subtype requires utter attention, as a large group of S1P receptor agonists/antagonists has been developed to target the receptor signalling in the central nervous system. For instance, FTY720, a S1P receptor agonist, can cross the blood-brain barrier and target NPSCs[133]. FTY720 advanced clinical trials were efficient for the treatment of multiple sclerosis. Restoration and protection of neural cells by FTY720 signalling has been shown for astrocytes and oligodendrocytes[132,134]. Moreover, FTY720 increased the viability and neurogenicity of irradiated NPSCs from the hippocampus[135], thus promising to serve as a healing agent for neurological diseases[133] (Figure 3).
Involvement of the SphK/S1P signalling axis in CSC functioning has been recently investigated in several malignancies, including glioblastoma[136], melanoma[137], hepatocellular carcinoma[138], and breast adenocarcinoma[139,140]. Considering the established role of sphingolipid signalling in mammary carcinomas, this study addressed the role of S1P receptors only in breast CSCs.
According to a cancer progenitor theory, mammary cancers originate from a small population of tumour-initiating cells. Marked by strong survival characteristics and a high level of heterogeneity, CSCs yield the majority of cancers through continuous self-renewal and very limited differentiation. CSCs have been reported to utilize similar molecular mechanisms as embryonic and normal adult stem cells. For instance, CSC self-renewal capacity has been associated with Notch, Hedgehog and Wnt signalling pathways[139]. Sphingolipid and particularly the S1P receptor signalling network has been recently explored in breast CSC models[140,141].
The stimulatory role of S1P and its effect on CSC proliferation was tested after the treatment of breast cancer cells with environmental carcinogens phthalate and benzyl butyl phthalate. These agents activate aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that is known to regulate quiescence, self-renewal, and differentiation of HSCs[142]. Activated AhR stimulated SphK1/S1P/S1PR3 signalling and promoted CSC-induced metastasis in vivo[141]. The study suggests that toxic agents and AhR triggered epigenetic activity (histone modification) in CSCs, which in turn, induced transcriptional activation of S1Pr3. Increased release of S1P was also observed because of SphK1 activation. S1P3 knockdown strongly decreased CD44high/CD24low (supposedly stem) MCF-7 cell populations[141].
Another group used different CSC markers and demonstrated a key regulatory role of S1P3 in mammary CSCs[140]. S1P enhanced the mammosphere-forming capacity of aldehyde dehydrogenase (ALDH)-positive CSCs via S1P3 and associated induction of the Notch signalling pathway. SphK1-overexpressing CSCs demonstrated an increased ability to develop tumors in nude mice in vivo. The tumorigenicity of these CSCs was also blocked by S1P3 knockdown and the specific S1P3 antagonists TY52156 and CAY10444[140]. The study detected high expression levels of S1P3, but lower S1P2 in the ALDH-positive CSC population. S1P activated Notch-dependent proliferation, employing ligand-independent activation of Notch via p38MAPK[140]. Notably, breast cancer patient-derived CSCs contained SphK1+/ALDH1+ cells or S1PR3+/ALDH1+ cells[140], indicating a leading role for this receptor in the maintenance of self-renewal potential.
Conclusively, inhibition of S1P3 signaling seems like an attractive clinical target in the treatment of breast cancers. One of the S1P receptor inhibitors, FTY720, might be a beneficial clinical agent. FTY720 can provoke global cytoskeletal change that results in deformed and decreased filopodia formation, reduced expression of integrins, apoptosis, decreased cancer cell adhesion, and prevention of metastasis[143]. These diverse multifunctional effects of FTY720 suggest an ability to interact with more than one specific target in tested cells (Figure 3). Thus, the exact mechanisms of FTY720 signaling was not tested in breast CSCs. FTY720 reactivated expression of the silenced estrogen receptor α(ERα) and sensitized them to tamoxifen, the widely used chemotherapy agent in mammary cancer patients[144]. However, the potential interaction of FTY720 and tamoxifen signaling remains unclear in CSCs. Tamoxifen is the tissue-specific ER agonist/antagonist/modulator shown to inhibit proliferation of ER-positive breast cancer cells. However, prolonged tamoxifen treatment up-regulates Wnt signaling and promotes survival of CSCs. Notably, ER signaling and tamoxifen resistance were mediated by SphK1/S1P3 receptor signaling in MCF-7 cells[11]. Moreover, estrogen was found to regulate breast CSC numbers through the FGF/Tbx3 signaling pathway, which is also responsible for the regulation of normal embryonic breast stem cell function[145]. An additive effect of tamoxifen and FTY720 in mammary CSCs remains to be explored in future studies.
The SphK/S1P receptor network has emerged as a key mediator of stem cell proliferation, survival and differentiation. The essential function of S1P receptor(s) for vascular and neural development has been proven in genetic knockout mice[146]. Considering the very high survival capacity of stem/progenitor cells, the activation of SphK/S1P signaling in normal progenitor and CSCs seems highly likely. S1P regulates cell proliferation and survival mainly through increased phosphorylation of p42/44-MAPK/Erk1/2 and PI3K/Akt, the two major chain reaction arms responsible for anti-apoptotic effects (Figures 2 and 3). In neurodegenerative disease, the S1P receptor agonist FTY720 may exert protective effects on oligodendrocyte survival, counteracting ceramide-induced apoptosis[103,147] (Figure 3). The role of SphK/S1P receptor signaling in the regulation of normal progenitor function looks very attractive. SphK/S1P/S1P receptor signaling should be explored as a promising strategy to promote tissue regeneration in acute myocardial infarction, muscular degeneration, and various neurological pathologies. While induction of SphK/S1P signaling might be useful to boost regeneration and survival of normal stem/progenitor cells, inhibition of the SphK/S1P-dependent survival pathway should be considered for cancer treatment/prevention[1,11,108,140]. Suggesting potential useful application of S1P receptor inhibitors in various CSCs, an increase in SphK/S1P3 signaling correlated with poor prognosis in breast cancer patients[1,11] and promoted mammary CSCs expansion[140,141].
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Saeki K, Tanabe S, Wakao H S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1651] [Cited by in RCA: 1678] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 2. | Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 3. | Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487-52492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Song DD, Zhou JH, Sheng R. Regulation and function of sphingosine kinase 2 in diseases. Histol Histopathol. 2018;33:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 7. | Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7:e38941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 409] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 10. | Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Sukocheva OA. Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming. Int J Mol Sci. 2018;19:pii: E420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Kihara A. Sphingosine 1-phosphate is a key metabolite linking sphingolipids to glycerophospholipids. Biochim Biophys Acta. 2014;1841:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Schulze H, Sandhoff K. Sphingolipids and lysosomal pathologies. Biochim Biophys Acta. 2014;1841:799-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Saba JD. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun. 1998;242:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Waters C, Sambi B, Kong KC, Thompson D, Pitson SM, Pyne S, Pyne NJ. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem. 2003;278:6282-6290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | El-Shewy HM, Johnson KR, Lee MH, Jaffa AA, Obeid LM, Luttrell LM. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J Biol Chem. 2006;281:31399-31407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758-7768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Sukocheva OA, Wang L, Albanese N, Pitson SM, Vadas MA, Xia P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol. 2003;17:2002-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Weissman IL. Stem cells are units of natural selection for tissue formation, for germline development, and in cancer development. Proc Natl Acad Sci USA. 2015;112:8922-8928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Zou T, Fan J, Fartash A, Liu H, Fan Y. Cell-based strategies for vascular regeneration. J Biomed Mater Res A. 2016;104:1297-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Jaramillo-Ferrada PA, Wolvetang EJ, Cooper-White JJ. Differential mesengenic potential and expression of stem cell-fate modulators in mesenchymal stromal cells from human-term placenta and bone marrow. J Cell Physiol. 2012;227:3234-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat Cell Biol. 2016;18:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Melchiorri AJ, Bracaglia LG, Kimerer LK, Hibino N, Fisher JP. In Vitro Endothelialization of Biodegradable Vascular Grafts Via Endothelial Progenitor Cell Seeding and Maturation in a Tubular Perfusion System Bioreactor. Tissue Eng Part C Methods. 2016;22:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Tremblay JR, LeBon JM, Luo A, Quijano JC, Wedeken L, Jou K, Riggs AD, Tirrell DA, Ku HT. In Vitro Colony Assays for Characterizing Tri-potent Progenitor Cells Isolated from the Adult Murine Pancreas. J Vis Exp. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 848] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 32. | Smith GS, Kumar A, Saba JD. Sphingosine Phosphate Lyase Regulates Murine Embryonic Stem Cell Proliferation and Pluripotency through an S1P2/STAT3 Signaling Pathway. Biomolecules. 2013;3:351-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 34. | Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 35. | Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 36. | Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 703] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 37. | Ogle ME, Olingy CE, Awojoodu AO, Das A, Ortiz RA, Cheung HY, Botchwey EA. Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche. Stem Cells. 2017;35:1040-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 647] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 39. | Kim C, Schneider G, Abdel-Latif A, Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ, Kucia M, Ratajczak MZ. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells--implications for tissue regeneration. Stem Cells. 2013;31:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M, Proia RL, Steinman L, Han MH, Hla T. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523:342-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 669] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 42. | Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D. Specific effects of microRNAs on the plant transcriptome. Dev Cell. 2005;8:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1116] [Cited by in RCA: 1023] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 43. | Arnon TI, Xu Y, Lo C, Pham T, An J, Coughlin S, Dorn GW, Cyster JG. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int J Neurosci. 2013;123:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367-29373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 46. | Mendelson K, Evans T, Hla T. Sphingosine 1-phosphate signalling. Development. 2014;141:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 47. | Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest. 2014;124:4823-4828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Williams PA, Stilhano RS, To VP, Tran L, Wong K, Silva EA. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P). PLoS One. 2015;10:e0123437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Poitevin S, Cussac D, Leroyer AS, Albinet V, Sarlon-Bartoli G, Guillet B, Hubert L, Andrieu-Abadie N, Couderc B, Parini A. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc Res. 2014;103:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Hu Y, Belyea BC, Li M, Göthert JR, Gomez RA, Sequeira-Lopez ML. Identification of cardiac hemo-vascular precursors and their requirement of sphingosine-1-phosphate receptor 1 for heart development. Sci Rep. 2017;7:45205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Svetlov SI, Sautin YY, Crawford JM. EDG receptors and hepatic pathophysiology of LPA and S1P: EDG-ology of liver injury. Biochim Biophys Acta. 2002;1582:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, Apohan E, Sentelle RD, Smith C, Gault CR. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941-5952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Bonder CS, Sun WY, Matthews T, Cassano C, Li X, Ramshaw HS, Pitson SM, Lopez AF, Coates PT, Proia RL. Sphingosine kinase regulates the rate of endothelial progenitor cell differentiation. Blood. 2009;113:2108-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Roato I, Ferracini R. Cancer Stem Cells, Bone and Tumor Microenvironment: Key Players in Bone Metastases. Cancers (Basel). 2018;10:pii: E56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Kimura T, Boehmler AM, Seitz G, Kuçi S, Wiesner T, Brinkmann V, Kanz L, Möhle R. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478-4486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, Aoki S, Yatomi Y, Takuwa Y, Nagawa H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res. 2006;130:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Li MH, Sanchez T, Yamase H, Hla T, Oo ML, Pappalardo A, Lynch KR, Lin CY, Ferrer F. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett. 2009;276:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Brühl ML, Tirniceriu A, Gaertner F. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165-2181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Vito CD, Hadi LA, Navone SE, Marfia G, Campanella R, Mancuso ME, Riboni L. Platelet-derived sphingosine-1-phosphate and inflammation: from basic mechanisms to clinical implications. Platelets. 2016;27:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989;58:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 713] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 62. | Grounds MD. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Donati C, Cencetti F, Bruni P. Sphingosine 1-phosphate axis: a new leader actor in skeletal muscle biology. Front Physiol. 2013;4:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 397] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 65. | Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol Biol Cell. 2010;21:1111-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 66. | Bernacchioni C, Cencetti F, Blescia S, Donati C, Bruni P. Sphingosine kinase/sphingosine 1-phosphate axis: a new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet Muscle. 2012;2:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Bruno G, Cencetti F, Bernacchioni C, Donati C, Blankenbach KV, Thomas D, Meyer Zu Heringdorf D, Bruni P. Bradykinin mediates myogenic differentiation in murine myoblasts through the involvement of SK1/Spns2/S1P2 axis. Cell Signal. 2018;45:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 958] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 69. | Frimm Cde C, Sun Y, Weber KT. Wound healing following myocardial infarction in the rat: role for bradykinin and prostaglandins. J Mol Cell Cardiol. 1996;28:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Ricciardolo FLM, Folkerts G, Folino A, Mognetti B. Bradykinin in asthma: Modulation of airway inflammation and remodelling. Eur J Pharmacol. 2018;827:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2005;19:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Sassoli C, Nosi D, Tani A, Chellini F, Mazzanti B, Quercioli F, Zecchi-Orlandini S, Formigli L. Defining the role of mesenchymal stromal cells on the regulation of matrix metalloproteinases in skeletal muscle cells. Exp Cell Res. 2014;323:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem. 2002;277:42997-43001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 76. | Meacci E, Donati C, Farnararo M, Bruni P. Sphingosine 1-phosphate signal transduction in muscle cells. Ital J Biochem. 2003;52:25-27. [PubMed] |
| 77. | Fortier M, Figeac N, White RB, Knopp P, Zammit PS. Sphingosine-1-phosphate receptor 3 influences cell cycle progression in muscle satellite cells. Dev Biol. 2013;382:504-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Castaldi A, Chesini GP, Taylor AE, Sussman MA, Brown JH, Purcell NH. Sphingosine 1-phosphate elicits RhoA-dependent proliferation and MRTF-A mediated gene induction in CPCs. Cell Signal. 2016;28:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Fukui H, Terai K, Nakajima H, Chiba A, Fukuhara S, Mochizuki N. S1P-Yap1 signaling regulates endoderm formation required for cardiac precursor cell migration in zebrafish. Dev Cell. 2014;31:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Araya R, Eckardt D, Maxeiner S, Krüger O, Theis M, Willecke K, Sáez JC. Expression of connexins during differentiation and regeneration of skeletal muscle: functional relevance of connexin43. J Cell Sci. 2005;118:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Squecco R, Sassoli C, Nuti F, Martinesi M, Chellini F, Nosi D, Zecchi-Orlandini S, Francini F, Formigli L, Meacci E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol Biol Cell. 2006;17:4896-4910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Saba JD, de la Garza-Rodea AS. S1P lyase in skeletal muscle regeneration and satellite cell activation: exposing the hidden lyase. Biochim Biophys Acta. 2013;1831:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | de la Garza-Rodea AS, Baldwin DM, Oskouian B, Place RF, Bandhuvula P, Kumar A, Saba JD. Sphingosine phosphate lyase regulates myogenic differentiation via S1P receptor-mediated effects on myogenic microRNA expression. FASEB J. 2014;28:506-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Gangoiti P, Bernacchioni C, Donati C, Cencetti F, Ouro A, Gómez-Muñoz A, Bruni P. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie. 2012;94:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 729] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 86. | Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 87. | Hashimoto Y, Matsuzaki E, Higashi K, Takahashi-Yanaga F, Takano A, Hirata M, Nishimura F. Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Mol Cell Biochem. 2015;401:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Shen H, Zhou E, Wei X, Fu Z, Niu C, Li Y, Pan B, Mathew AV, Wang X, Pennathur S. High density lipoprotein promotes proliferation of adipose-derived stem cells via S1P1 receptor and Akt, ERK1/2 signal pathways. Stem Cell Res Ther. 2015;6:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 90. | Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 874] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 91. | Cebola I, Rodríguez-Seguí SA, Cho CH, Bessa J, Rovira M, Luengo M, Chhatriwala M, Berry A, Ponsa-Cobas J, Maestro MA. TEAD and YAP regulate the enhancer network of human embryonic pancreatic progenitors. Nat Cell Biol. 2015;17:615-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 92. | Serafimidis I, Rodriguez-Aznar E, Lesche M, Yoshioka K, Takuwa Y, Dahl A, Pan D, Gavalas A. Pancreas lineage allocation and specification are regulated by sphingosine-1-phosphate signalling. PLoS Biol. 2017;15:e2000949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952-6960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Rius RA, Edsall LC, Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997;417:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Tincer G, Mashkaryan V, Bhattarai P, Kizil C. Neural stem/progenitor cells in Alzheimer’s disease. Yale J Biol Med. 2016;89:23-35. [PubMed] |
| 96. | Tashiro S, Nishimura S, Iwai H, Sugai K, Zhang L, Shinozaki M, Iwanami A, Toyama Y, Liu M, Okano H. Functional Recovery from Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training in Mice with Chronic Spinal Cord Injury. Sci Rep. 2016;6:30898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88:1026-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 99. | Anelli V, Bassi R, Tettamanti G, Viani P, Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem. 2005;92:1204-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS One. 2011;6:e23905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 101. | Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Bieberich E. There is more to a lipid than just being a fat: sphingolipid-guided differentiation of oligodendroglial lineage from embryonic stem cells. Neurochem Res. 2011;36:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Spohr TC, Dezonne RS, Nones J, Dos Santos Souza C, Einicker-Lamas M, Gomes FC, Rehen SK. Sphingosine 1-phosphate-primed astrocytes enhance differentiation of neuronal progenitor cells. J Neurosci Res. 2012;90:1892-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Blanc CA, Grist JJ, Rosen H, Sears-Kraxberger I, Steward O, Lane TE. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. Am J Pathol. 2015;185:2819-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Meng H, Yuan Y, Lee VM. Loss of sphingosine kinase 1/S1P signaling impairs cell growth and survival of neurons and progenitor cells in the developing sensory ganglia. PLoS One. 2011;6:e27150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 442] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 109. | Madhunapantula SV, Hengst J, Gowda R, Fox TE, Yun JK, Robertson GP. Targeting sphingosine kinase-1 to inhibit melanoma. Pigment Cell Melanoma Res. 2012;25:259-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res. 2009;50 Suppl:S272-S276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Dobrowsky RT, Carter BD. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann N Y Acad Sci. 1998;845:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61:205-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 113. | Barrett GL, Bartlett PF. The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc Natl Acad Sci USA. 1994;91:6501-6505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 290] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 114. | Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 586] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 115. | Bonni A, Ginty DD, Dudek H, Greenberg ME. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6:168-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 242] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 116. | Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 974] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 117. | Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 347] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 118. | Saini HS, Coelho RP, Goparaju SK, Jolly PS, Maceyka M, Spiegel S, Sato-Bigbee C. Novel role of sphingosine kinase 1 as a mediator of neurotrophin-3 action in oligodendrocyte progenitors. J Neurochem. 2005;95:1298-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Johnson JR, Chu AK, Sato-Bigbee C. Possible role of CREB in the stimulation of oligodendrocyte precursor cell proliferation by neurotrophin-3. J Neurochem. 2000;74:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 998] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 121. | Bu S, Asano Y, Bujor A, Highland K, Hant F, Trojanowska M. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 2010;62:2117-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Hida H, Nagano S, Takeda M, Soliven B. Regulation of mitogen-activated protein kinases by sphingolipid products in oligodendrocytes. J Neurosci. 1999;19:7458-7467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Fatatis A, Miller RJ. Platelet-derived growth factor (PDGF)-induced Ca2+ signaling in the CG4 oligodendroglial cell line and in transformed oligodendrocytes expressing the beta-PDGF receptor. J Biol Chem. 1997;272:4351-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Soliven B, Ma L, Bae H, Attali B, Sobko A, Iwase T. PDGF upregulates delayed rectifier via Src family kinases and sphingosine kinase in oligodendroglial progenitors. Am J Physiol Cell Physiol. 2003;284:C85-C93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | Wang F, Buckley NE, Olivera A, Goodemote KA, Su Y, Spiegel S. Involvement of sphingolipids metabolites in cellular proliferation modulated by ganglioside GM1. Glycoconj J. 1996;13:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Su Y, Rosenthal D, Smulson M, Spiegel S. Sphingosine 1-phosphate, a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1994;269:16512-16517. [PubMed] |
| 127. | Shatrov VA, Lehmann V, Chouaib S. Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-kappa B in U937 cells. Biochem Biophys Res Commun. 1997;234:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196-14201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 339] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 129. | Takeshita A, Watanabe A, Takada Y, Hanazawa S. Selective stimulation by ceramide of the expression of the alpha isoform of retinoic acid and retinoid X receptors in osteoblastic cells. A role of sphingosine 1-phosphate-mediated AP-1 in the ligand-dependent transcriptional activity of these receptors. J Biol Chem. 2000;275:32220-32226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Im DS, Clemens J, Macdonald TL, Lynch KR. Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (Edg-8): structure-activity relationship of sphingosine1-phosphate receptors. Biochemistry. 2001;40:14053-14060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 132. | Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 133. | Tan B, Luo Z, Yue Y, Liu Y, Pan L, Yu L, Yin Y. Effects of FTY720 (Fingolimod) on Proliferation, Differentiation, and Migration of Brain-Derived Neural Stem Cells. Stem Cells Int. 2016;2016:9671732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 494] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 135. | Stessin AM, Gursel DB, Schwartz A, Parashar B, Kulidzhanov FG, Sabbas AM, Boockvar J, Nori D, Wernicke AG. FTY720, sphingosine 1-phosphate receptor modulator, selectively radioprotects hippocampal neural stem cells. Neurosci Lett. 2012;516:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 136. | Mahajan-Thakur S, Bien-Möller S, Marx S, Schroeder H, Rauch BH. Sphingosine 1-phosphate (S1P) signaling in glioblastoma multiforme-A systematic review. Int J Mol Sci. 2017;18:pii: E2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 137. | Mukherjee N, Lu Y, Almeida A, Lambert K, Shiau CW, Su JC, Luo Y, Fujita M, Robinson WA, Robinson SE. Use of a MCL-1 inhibitor alone to de-bulk melanoma and in combination to kill melanoma initiating cells. Oncotarget. 2017;8:46801-46817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Luo J, Wang P, Wang R, Wang J, Liu M, Xiong S, Li Y, Cheng B. The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget. 2016;7:9525-9537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 139. | Takebe N, Ivy SP. Controversies in cancer stem cells: targeting embryonic signaling pathways. Clin Cancer Res. 2010;16:3106-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 140. | Hirata N, Yamada S, Shoda T, Kurihara M, Sekino Y, Kanda Y. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat Commun. 2014;5:4806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 141. | Wang YC, Tsai CF, Chuang HL, Chang YC, Chen HS, Lee JN, Tsai EM. Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget. 2016;7:29563-29576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 142. | Gasiewicz TA, Singh KP, Bennett JA. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci. 2014;1310:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 143. | Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410-1419. [PubMed] |
| 144. | Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4:e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 145. | Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci USA. 2010;107:21737-21742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 146. | Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113-11121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 576] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 147. | Bieberich E. Smart drugs for smarter stem cells: making SENSe (sphingolipid-enhanced neural stem cells) of ceramide. Neurosignals. 2008;16:124-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |