Published online May 26, 2018. doi: 10.4252/wjsc.v10.i5.43
Peer-review started: March 28, 2018
First decision: April 9, 2018
Revised: April 26, 2018
Accepted: May 9, 2018
Article in press: May 10, 2018
Published online: May 26, 2018
Processing time: 58 Days and 11.7 Hours
The use of stem cells as carriers for therapeutic agents is an appealing modality for targeting tissues or organs of interest. Combined delivery of cells together with various information molecules as therapeutic agents has the potential to enhance, modulate or even initiate local or systemic repair processes, increasing stem cell efficiency for regenerative medicine applications. Stem-cell-mediated delivery of genes, proteins or small molecules takes advantage of the innate capability of stem cells to migrate and home to injury sites. As the native migratory properties are affected by in vitro expansion, the existent methods for enhancing stem cell targeting capabilities (modified culture methods, genetic modification, cell surface engineering) are described. The role of various nanoparticles in equipping stem cells with therapeutic small molecules is revised together with their class-specific advantages and shortcomings. Modalities to circumvent common challenges when designing a stem-cell-mediated targeted delivery system are described as well as future prospects in using this approach for regenerative medicine applications.
Core tip: The capability of stem cells to mobilize, home and target to inflammatory sites justifies their use as delivery agents for regenerative medicine purposes. Cell and membrane engineering techniques can be used to increase the selective targeting potential of stem cells. Gene therapy and nanoparticle-mediated small-molecule delivery of informational cues have the potential to increase the efficiency of clinically relevant stem-cell-based regenerative therapies.
- Citation: Labusca L, Herea DD, Mashayekhi K. Stem cells as delivery vehicles for regenerative medicine-challenges and perspectives. World J Stem Cells 2018; 10(5): 43-56
- URL: https://www.wjgnet.com/1948-0210/full/v10/i5/43.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i5.43
Stem cells, the natural reservoir for growth, development and repair in all multicellular organisms, have been the object of intense scrutiny in the last several decades for scientific reasons, but most of all, for their potential applications in biology and medicine. Stem cells are defined by their capability to self-renew and to differentiate into more specialized progeny according to their degree of potency[1]. The emerging domain of regenerative medicine (RM) aims to make possible the complete functional and structural restoration of tissues, organs or even bodily systems. Due to their unique properties in modulating local and systemic reparatory processes, stem cells are employed by RM as main the therapeutic tool[2]. Basic and preclinical research involving (stem) cell therapy to fight acute or chronic degenerative disease in various medical fields is currently underway. Stem-cell-based tissue engineering applications aim to produce implantable bio-substitutes that could waive the need for costly tissue and organ transplantation[3,4]. Some of the products of this new, research-intensive medical field are already in the stage of clinical trials, which promises to deliver revolutionary therapies in the near future.
Different types of stem cells are currently tested for RM applications: Embryonic stem cells (ESCs)[5] adult stem cells (ASCs)[6] or induced pluripotent stem cells (iPSCs)[7], each of them with specific advantages and risks. An outline of stem cell type-dependent advantages and shortcomings relative to their use as therapeutic agents is beyond the scope of this review; the reader is referred to excellent works in this respect[8,9].
Dependent on their type and therapeutic application, stem cells are expected to exert a certain effect, be it in recomposing the anatomy and/or function of a given structure, in restoring dysfunctional biological pathways or in modulating the local or systemic immune response. Stem-cell-based TE of various tissues (such as bone, cartilage, neural, cardiac or skeletal muscle and so on) is based on the cell capability to generate tissue specific progenies that directly recompose the given structure. TE strategies are largely based on directed differentiation and structural participation of ESCs, iPSCs or mesenchymal stem cells (MSCs). Due to their large availability, MSCs are the most commonly used ASCs for RM applications. MSCs were shown to release cytokines and growth factors (GFs) that exert paracrine effects upon transplantation within an injured structure, contributing to tissue repair by recruiting local cells to induce repair[10]. The expression of bioactive molecules with multiple trophic (regenerative) and immunomodulatory effects has prompted a call for changing the MSC denomination in medicinal signalling cells to more accurately reflect their potential for acting as therapeutic drugs[11]. Stem-cell-derived microvesicles containing mRNA, microRNA and proteins and released as exosomes into the intercellular milieu as a modality of cell-cell communication are increasingly recognized as potent therapeutic agents. ESC- and MSC-derived exosomes that contribute to restraining tissue injury and induce cell cycle re-entry of resident cells, leading to tissue self-repair, are being tested for different applications in cardiac, neural or musculoskeletal repair[12,13].
ESCs and MSCs were shown to home and engraft to the site of injury, facilitating tissue and organ repair following traumatic, degenerative, ischaemic or inflammatory processes[14,15]. The stem cell migratory potential enables the choice for systemic delivery whenever the direct, intra-lesion administration is invasive, implies associated morbidity or involves the situation of multiple and/or remote lesions[16]. In particular, MSCs are in focus as delivery vehicles due to their homing capabilities, low immunogenicity (not expressing class II histocompatibility molecules) and immune-modulatory capabilities. Recently, the use of stem cells as delivery agents for artificially produced molecules that exert a local or systemic therapeutic effect has emerged as another field of cell therapy application. In the following, a brief outline of MSC native and enhanced homing mechanisms, methods of loading cells with therapeutic agents and several current applications of stem-cell-based delivery will be presented.
Tissues and organs possess, at least to a certain extent, the capability to repair or regenerate. The natural repair mechanisms are based on activation of the intrinsic progenitor population, trans-differentiation of local adult elements and the capability to recruit and home circulating stem cells[17]. Homing has been defined as the cell capability to arrest in the blood stream and localize and migrate through the endothelial walls of blood vessels within the tissue. This spontaneously occurring process enhances reparatory mechanisms, adding both cellular stock as well as signalling molecules, and normally leads to restoration of local structural and physiological parameters. The capability of self-repair can be overwhelmed by the severity of the injury combined with local or systemic particularities such as inflammation, disease or ageing.
The use of stem cells as therapeutic agents is expected to increase or substitute regenerative processes. The use of stem cells as “biological regenerative supplementation” is largely based on their natural capability to mobilize, migrate and home. The mechanisms for cell migration and “nesting” to the sites of injuries have been described mostly in relation to MSCs, particularly regarding their migration to the bone marrow and other tissues. When mobilized from the host or therapeutically administered, similarly to leucocytes, MSCs first reach the blood stream, entering the systemic venous or arterial circulation. Reaching the target, the cells decelerate and come in contact with the endothelial walls by rolling and tethering. In this second step, MSC G-coupled protein receptors are activated followed by their arrest on the endothelial membrane within the target tissue by means of integrin receptor activation (third step)[18]. Transmigration through both the endothelium and the underlying basal extracellular matrix (ECM) membrane represent the fourth and final step of homing. The process normally takes a few hours and results in transient retention of stem cells within the tissue. The navigation process of homing only implies cell anchorage and transmigration from the blood stream to the tissue. Depending on the stem cell type and tissue characteristics, this process can be followed by division of the migrated cells, having as a consequence their engraftment and, eventually, tissue repopulation[19]. Homing is based on several constitutive abilities of stem cells to respond to external cues that enable their trafficking, guidance and migration. For therapeutic purposes, both stem cells as well as the external cues that govern homing can be modulated to enhance the targeting process and optimize the expected results.
Stem cells sense and are able to move according to a chemoattractant gradient by means of amoeboid cell migration. This highly conserved process, termed “chemotaxis” or “interstitial migration”, can take place independently of blood flow. Chemoattractant gradients within the extracellular space or mechanical signals guide cell movement by directing cellular actin polymerization. Different cell types can migrate (such as leucocytes, progenitor and stem cells or metastatic cancer cells) displaying specific changes in their focal adhesion, myosin-based contractility and actin polymerization[20]. In naturally occurring circumstances, the first steps of the migration of progenitor cells require their mobilization by disruption of the ECM as well as cell adhesion to matrix proteins by means of proteolytic enzymes released by the injury and/or local inflammatory processes[21]. In turn, stem cells release proteases that contribute to ECM remodelling, also facilitating the migratory process. MSCs were shown to express several key components of the fibrinolytic cascade (including urokinase plasminogen activator receptor (uPAR) and plasminogen activator inhibitor (PAI-10) of the fibrinolytic system) known to exert crucial roles in cell migration, growth factor bioavailability during inflammation, tissue regeneration and cancer[22]. The involvement of an autocrine mechanism based on heat shock protein 70 (HSP70) released by murine mesangioblast interaction with Toll-like receptor 4 (TLR4) and CD91 has been proposed as another mechanism responsible for the stem cell ability to transverse the ECM and stimulate migration[23]. Adhesion molecules (such as E and P selectins) play an important role in the homing of haematopoietic stem cells (HSCs), being involved in the first step of tethering and rolling of those cells along the endothelial wall. Integrins such as CD49d/CD29 (α4β1 or VLA-4) and CD11a/CD18 (αLβ2 or LFA-1) are involved in adhesion of HSCs to the endothelium and trans-endothelial migration[24]. VLA-4 binds to vascular cell adhesion molecule 1 (VCAM-1) within the endothelium and is functionally involved in MSC homing. MSCs express a large variety of chemokine membrane receptors that enable their migration towards wounds, inflammation and malignant-site-released chemokine gradients. It should be noted that chemokine receptors were shown to be species dependent, which is of importance when translating results from animal studies to clinical applications. For cultured MSCs, isolation and expansion methods are known to strongly influence the chemokine receptor repertoire inviting a thorough assessment when such cells are sought for targeting therapies[25]. Several chemokine axes are known to regulate the homing of a large variety of stem cells. The CXCL12 (also known as stromal derived factor 1α, SDF-1α)-CXCR4 axis may represent a general mechanism for the chemoattraction of MSCs, haematopoietic stem cells, and neural and endothelial progenitors to injury sites. CXCL12-CXCR4 is constitutively expressed in several cell populations such as dermal fibroblasts, endothelial cells or pericytes, increasing consistently during tissue injury. CXCR4 and CXCR7 receptors that bind SDF-1α have been detected on the surface of bone-marrow-derived MSCs and adipose-derived stem cells (ASDCs) that use an SDF1-α gradient to migrate to the site of acute and chronic injuries[26]. Constitutively expressed on ESCs, CXCR4 is a major contributor to organogenesis during development. SDF-1α/CXCR4 is also involved in pathological processes such as cancer stem cell migration and cancer metastasis[27]. Other chemokine axes such as CCL27-CCR10, CCL5-CCR5 and the more skin-specific CCL21-CCR7 are involved in natural processes of stem cell migration and homing. Different methods of enhancing chemokine-based gradient targeting are currently under scrutiny for regenerative purposes (see below)[28].
Targeting injured or diseased tissues by stem cells largely depends on cell characteristics as well as on the existence of a chemoattractant gradient to guide their trafficking and homing. Stem cells and particularly MSCs are shown to possess migratory and targeting capabilities; however, only a small portion of therapeutically administered cells can home and engraft within the injured tissue[29]. Several strategies addressing cells such as modified culture conditions, specific preconditioning or manipulation before transplantation are currently being tested with the purpose of increasing their migratory capabilities. Methods that modify external conditions such as improved delivery methods or modified route of administration contribute to increasing the number of cells that reach their target.
Depending on their tissue of origin and donor age and in the absence of natural stimuli, MSCs express low amounts of chemokine receptors[30]. In vitro expansion, which is required for obtaining a clinically relevant number of cells, further decreases the number of these receptors. Culturing stem cells under hypoxic conditions was shown to increase CXCR4, CXCR7 and SDF-1 expression by means of a hypoxia inducible factor-1α (HIF-1α) mechanism[31]. Hypoxia can increase chemokine receptor expression, induce the differential expression of MMPs, reduce reactive oxygen species by reduced mitochondrial respiration, and induce Notch signalling pathway-all factors known to be involved in sustaining proliferative and migratory capabilities in stem cells[32]. Some studies have raised concerns about the safety of MSCs cultured under hypoxic conditions[33], although other reports show that the method results in culture-expanded cells that grow faster and display an enhanced migratory capability while being non-oncogenic and retaining multipotency[34]. MSCs cultured at lower confluence were shown to possess superior migratory capabilities. It has been reported that highly confluent MSCs adapt to this condition by expressing higher amounts of tissue inhibitor of metalloproteinase-3 (TIMP-3), resulting in a decreased migratory potential[35].
Three-dimensional (3D) culture conditions were shown to increase differentiation and surface antigen expression, increasing their therapeutic potential in terms of cell viability and targeting capabilities[36]. Increased cell survival after transplantation and reduced replicative senescence was observed in spheroid cultured MSCs; however, a direct impact on the cell migratory potential and how this correlates with the drastic modification in the cell cytoskeleton under such conditions, needs to be further analysed[37].
An interesting approach exists for increasing CXCR4 expression in mouse bone marrow MSCs that have internalized magnetic nanoparticles (MNPs). MNP-loaded cells were shown to possess improved cell homing efficiency. MNP payload enabled cell tracking in vivo using magnetic resonance imaging (MRI), demonstrating this could be an efficient strategy for enhancing cell targeting and tracking capability[38].
performed by exposing cultured cells to various soluble molecules is used to improve stem cell homing. Concern exists about the gradual decrease of chemokine receptors in cultivated MSCs; therefore, preconditioning could not only counteract this phenomenon but also enhance their innate targeting capability. Increased expression of cytokine membrane receptors (CXCR4) could be obtained using a cocktail of factors added to the culture media. Fms-like tyrosine kinase (Flt-3) ligand, stem cell factor (SCF), interleukin (IL) and hepatocyte growth factor (HGF) were shown to rapidly increase CXCR4 expression in human foetal-derived bone marrow stem cells and to increase their homing potential within the bone marrow of sub-lethally irradiated NOD/SCID mice[39]. Conditioned medium from tumour necrosis factor alpha (TNFα) pre-stimulated cord-blood-derived stem cells were shown to enhance intravenously and intramuscularly administered epithelial progenitor cells (EPCs) in a model of hind limb ischaemia by a mechanism involving interleukin-6 (IL-6) and interleukin 8 (IL-8)[40].
Preconditioning stem cells with insulin growth factor-1 (IGF-1) or with SDF-1 were shown to improve their migratory and homing capability in vitro and in vivo[41]. Other small molecules such as inhibitors of glycogen synthase kinase-3β (GSK-3β) or erythropoietin combined with granulocyte–colony stimulating factor (G-CSF) were shown to enhance MSC migratory capability by enhancing CXCR4 and MMP expression or by stimulating the extracellular signal related kinase (ERK)1/2 pathway, respectively[42,43]. MSC preconditioning with oxytocin was shown to increase the expression of protein kinase B (PKB or Akt) and phospho-ERK1/2 together with other proteins and genes such as vascular endothelial growth factor, thrombospondin, tissue inhibitor of metalloproteinase-(TIMP-) 1, TIMP-2, TIMP-3, and MMP-2, increasing their therapeutic potential[44,45]. Histone deacetylase (HDAC) inhibitor valproic acid combined with lithium was shown to be effective in enhancing the MSC migratory ability in vitro by means of a HADC-CXCR4, GSK-3β-MMP9 stimulatory mechanism[46].
Another strategy to improve stem cell homing is to increase the expression of targeting molecules in therapeutic cells by gene manipulation. CXCR4 overexpression has been reported by several groups to show a variable efficiency in increasing the targeting potential of MSCs. Non-viral methods are preferred, especially considering potential clinical applications, but are notorious for having a low transfection efficiency. The use of several cationic liposomal agents (such as IBAfect, a polycationic liposomal transfection reagent) was shown to yield an improved transfection efficiency compared to that of adenoviral methods, resulting in a superior chemotactic index in transfected cord-blood-derived MSCs[47]. Overexpression of other chemokine receptors, such as CXCR7 and CXCR1, was shown to enhance the migratory and targeting properties in various stem cell populations[48].
Stem cell surface modification with rapid incorporation of recombinant CXCR4 protein on the membrane was shown to enhance stem cell migration towards an SDF-1 gradient[49]. Surface engineering aims to transiently modify cell membrane in order to improve their adhesion or endothelial transmigration. Several ingenious methods such as CD44 fucosylation to obtain P-selectin glycoprotein ligand-1 (PSGL-1) or HCELL on the surface of MSCs, biotinylation of MSC membranes or conjugating various antibodies against adhesion molecules [intercellular adhesion molecule (ICAM) or vascular adhesion molecule (VCAM-1)] were shown to increase the homing in surface-engineered cells[50].
Mode and route of administration is an important factor that influences therapeutic cell survival, migration and homing potential. Intravenous administration, the most commonly used modality of systemic cell delivery, poses the inconvenience of cell trapping within organs such as the lung, spleen or liver. This results in an important quantity of capillary-arrested cells and a decreased therapeutic effect if the target is located elsewhere. Pre-treatment of the host with vasodilatory substances or heparin administration pre-procedure was shown to diminish ADSC lung trapping and to increase hepatic targeting in a rat model of liver failure[51].
Stem cells, and particularly MSCs, can be genetically engineered to release therapeutic proteins for regenerative purposes, with various applications in treating monogenic diseases (such as muscular dystrophy or haemophilia) or even degenerative diseases (such as inflammatory arthritis). Even if viral transduction methods were preferred in the beginning for their superior transfection rate, the improved efficiency of newer non-viral methods support their use for therapeutic purposes. Lower immunogenicity, increased scalability, decreased toxicity and considerable versatility of non-viral methods compared to viral methods recommend the use of the former when considering clinical applications[52]. Physical methods such as electroporation and nucleofection, which use an electric pulse to ferry nucleic acids through the cell membrane or nuclear membrane, respectively, as well as ultrasound-based gene delivery are methods that require intensive protocol optimization due to decreased cell viability[53]. Chemical non-viral methods employ liposomes, dendrimers, inorganic nanoparticles, magnetic nanoparticles (MNPs) or polymers as transfection agents. High transfection efficiency was reported using poly (ethylenimine) (PEI) in combination with gold or silica nanoparticles[54,55].
Genetically engineered stem cells are under scrutiny for the treatment of several monogenic diseases. Using systemically delivered MSCs, a high level of therapeutic protein production can be obtained using stem-cell-mediated gene delivery, which can substitute for the abnormal gene function at the tissue and organ level. Hydroxyapatite- polylactic-polyglycolic acid (PLGA) composites can be coated with biomineralized collagen 1 in combination with autologous gene-engineered factor IX (hFIX). MSCs were shown to deliver a consistent amount of hFIX in a mouse model of haemophilia B[56]. Autologous HSCs transduced with a viral vector containing a healthy copy of the mutated gene were shown to improve lysosomal storage diseases such as metachromatic leukodystrophy (MLD) or mucopolysaccharidosis type I (MPS-I). The method allows production of the defective enzyme and cross correction of target cells in multiple target tissues[57].
Osteogenesis imperfecta (OI) represents a group of genetic disorders characterized by bone disease produced by missing or abnormal synthesis of type I collagen, with different levels of severity[58]. Adeno-associated virus vector MSCs from OI patients were shown to disrupt dominant-negative mutant COL1A1 collagen genes and to produce normal collagen similar to that of wild-type cells-a fact that could be used to develop a therapeutic platform for addressing this incurable and disabling disease[59].
A particular field of interest is to use genetically modified stem cells for inducing immune tolerance against antigens of interest in several autoimmune diseases (such as diabetes type I) or after organ or cell transplantation. Several strategies include transfection of a particular gene into HSCs or immunological precursor cells by educating the host immune system to recognize the therapeutic protein as “self”. Another possibility is to induce the expression of a therapeutic protein in antigen-presenting cells such as immature dendritic cells or B cells that will induce immune tolerance for the respective antigen[60].
A large portion of genetic engineering strategies aim to improve stem cell survival and homing potential during cell transplantation for various cell therapy applications. A particular field of therapeutic protein delivery intends, however, to use genetic manipulation for providing repair and regeneration enhancers or to substitute for congenitally absent or abnormal protein production. Stem-cell-mediated gene delivery for RM is sought as a method for enhancing stem cell survival and/or targeting capabilities (see above), to increase local or systemic reparative processes or for steering immunomodulatory processes. Musculoskeletal tissue regeneration, cardiac diseases and brain traumatic injury are among the several domains in which this method is currently being tested.
Autologous or allogeneic MSCs engineered to overexpress bone morphogenetic protein (BMP) was found to enable the repair of large bone defects in several animal models[61]. Systemically administered, viral transduced MSCs encoding BMP and vascular endothelial growth factor (VEGF) were able to promote the repair of large segmental bone defects in mice[62]. Scaffold-mediated locally delivered ADSCs overexpressing runx-2 were able to induce healing in large rat calvaria defects[63]. Bone marrow derived MSCS (BMSCs) cell sheets transfected with Lipofectamine 200 to overexpress antimiR-138 (a miRNA precursor) were shown to significantly increase osteogenesis in vitro. Forced expression of the transcription factor early growth response protein (Egr1)-programmed MSCs towards the tendon lineage promoted the formation of in vitro-engineered tendons, while Smad 8/BMP overexpressing MSCs locally delivered to injured Achilles tendons were shown to improve the histology and biomechanical properties[64,65]. However, caution needs to be exerted when assessing the therapeutic efficiency, as long-term results might differ from those of the immediate evaluation. MSCs engineered to overexpress basic fibroblast growth factor (bFGF) did not cause improvement in the histological appearance and biomechanical properties of the Achilles tendon 12 mo after transplantation in a rat model of tendon defect, questioning the overall impact of such therapies[66].
Cell sheets are versatile structures that can be easy manoeuvrable and accommodate a large variety of clinical defects[67]. MSCs overexpressing IL-8 binding protein (IL-BP8) were shown to decrease inflammation and enhance repair by means of enhanced vascularization in a rat model of global cardiac ischaemia[68]. ADSC sheets overexpressing VEGF were shown to reduce the infarct size and to improve cardiac functions to non-diseased levels in a rabbit model of cardiac infraction[69].
Adeno-associated virus (AAV)-transfected MSCs overexpressing IL-10 were proved to provide neuroprotection and enhance intravenously administered stem cell engraftment, improving brain recovery in a rat model of acute ischaemic stroke[70]. Human umbilical cord blood-derived mesenchymal stem cells (HUMBSCs) overexpressing VEGF were shown to reduce the clinical manifestation and the loss of dopaminergic neurons in the lesioned substantia nigra in hemi-parkinsonian rats. Enhanced HUMBSC differentiation to dopaminergic neurons was observed during this experiment, offering an improved modality for cell transplantation in Parkinson’s disease[71].
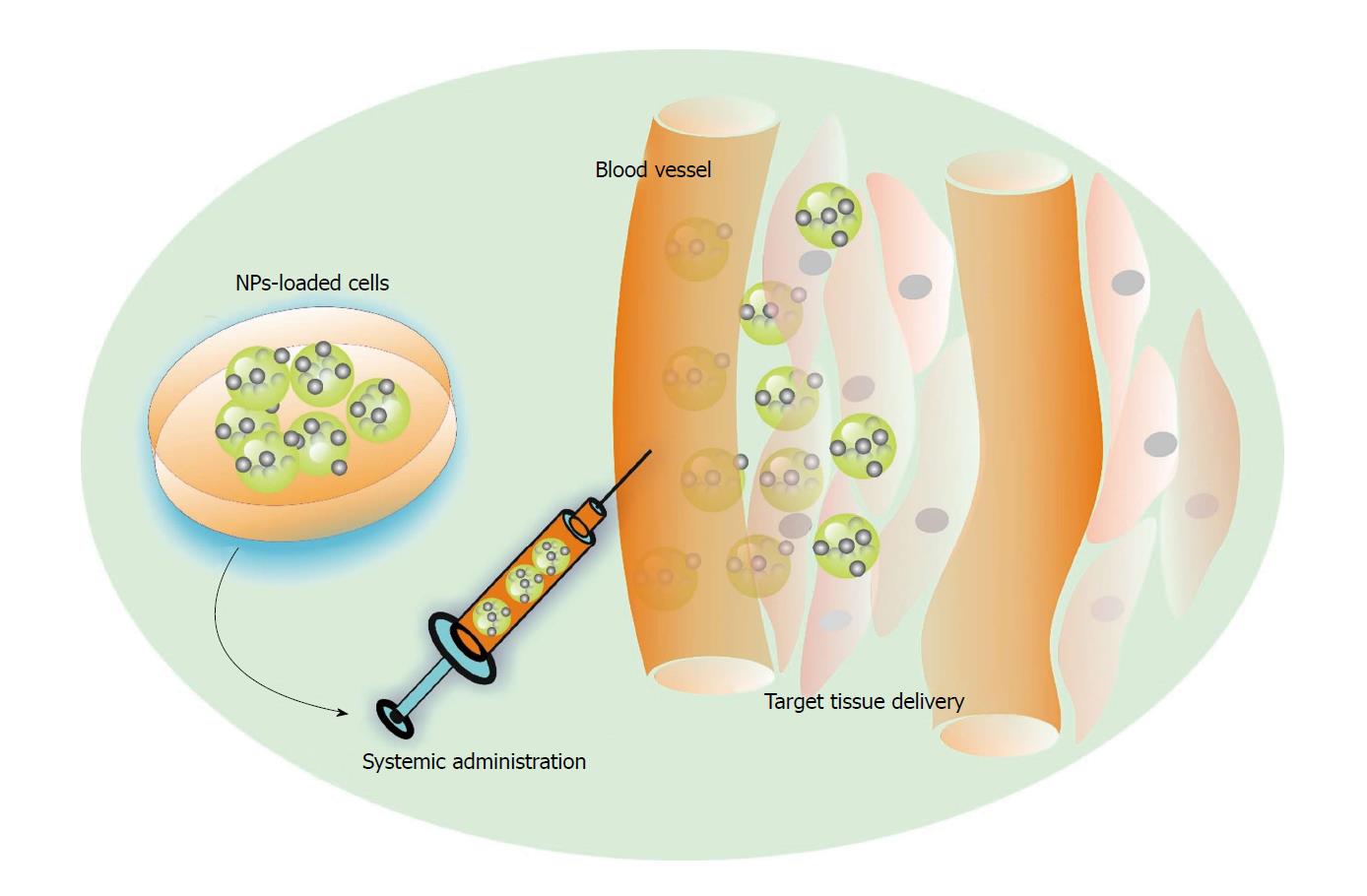
Genetic engineering of therapeutic cell populations can result in the release of only proteins that are intended to contribute to cell viability, proliferation, homing potential or to influence the regenerative process. Stem cells can also be engineered to deliver a larger variety of therapeutic molecules, making them veritable drug-delivery tools. Delivery of bioactive molecules is an important chapter for RM purposes. A group of information mediators and signalling molecules are grouped in three overlapping categories: (1) Mitogens (stimulate cell division); (2) growth factors (GFs) with multiple biological actions; and (3) morphogens (control the generation of tissue form) are crucial factors for initializing and sustaining regeneration and repair; therefore, the control of signalling molecules may potentially allow the control and modulation of regenerative processes. GFs are proteins or steroid hormones that stimulate cell growth, differentiation and healing[72]. Functional particularities of GF consist in their short-range diffusion through the extracellular matrix. To orchestrate regeneration, controlled local delivery coupled with precise timing is needed in order to obtain the desired action on target cells. Nanoparticles (NP) of different composition, shapes and physical properties are currently used to load native, primed or modified stem cells with information molecules intended to modulate regenerative processes. NP-drug complexes can be internalized by the cells or attached to their surface to be released at the target tissue following cell migration and targeted homing[73]. Such a procedure could be extended for enabling information-molecule-equipped stem cells to target regenerative sites (Figure 1).
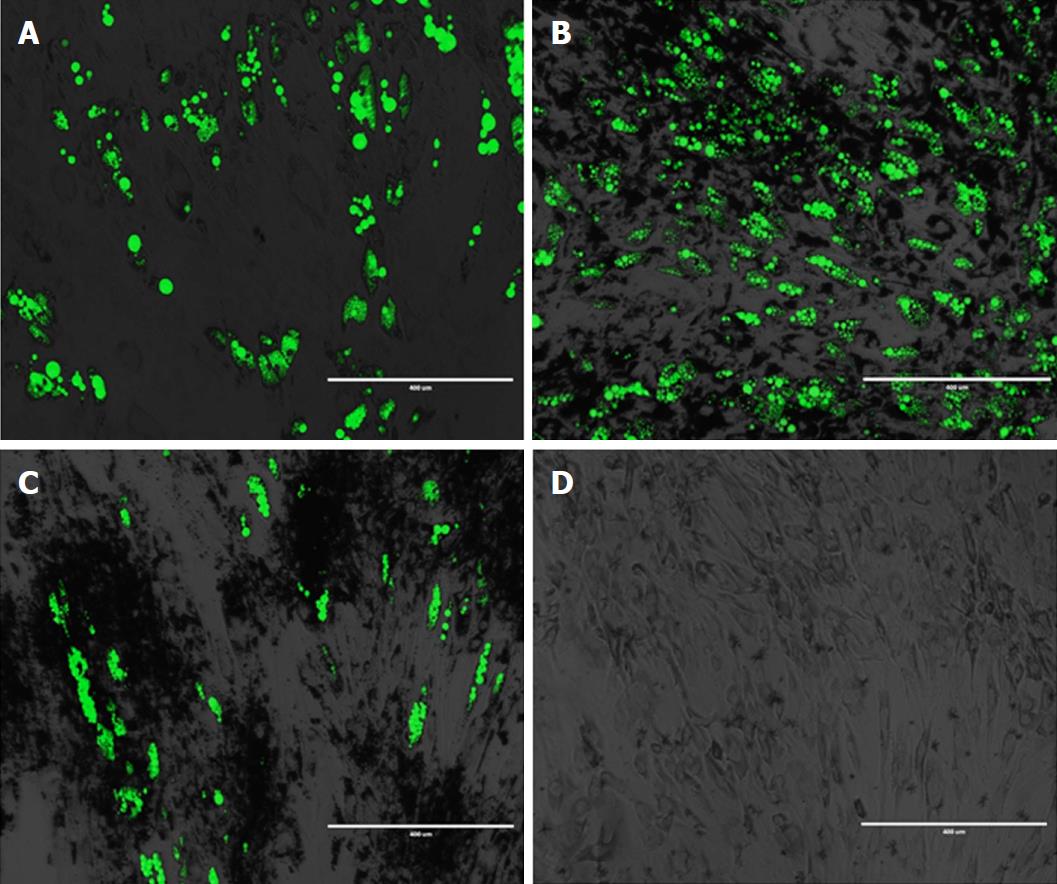
One of the most straightforward procedures is to load stem cells with NPs by simply adding them within the cell culture media, taking advantage of cell membrane mechanisms responsible for foreign body internalization (Figure 2). NP complexes are internalized by cells via endocytosis, a largely polymorphic process that depends on the cell type, particle size, composition, culture media formulation, electrical charge, and surface modifications-to name only some of the factors. NPs intended to be used for regenerative purposes must possess several obligatory characteristics such as (1) Biocompatibility, i.e., not being toxic to cells, tissues and organs and not eliciting an immune response at the local or systemic level; (2) biodegradability-capable of being decomposed by means of normal cellular metabolic pathways; and (3) stability of physical properties after surface modification; efficacy at therapeutic doses; chemical stability in physiological conditions[74]. Moreover, NP-loaded stem cells need to preserve critical stem cell characteristics such as proliferative, differentiation, and immune-modulatory as well as migratory and homing capabilities. Each type of NP-drug complex must be thoroughly characterized with regard to chemical and physical properties, effect on cell viability, phenotypic features, and migratory and homing potential after particle internalization.
Several classes of NPs, of which the most commonly used are briefly outlined below, can be employed for loading stem cells with small molecules, each of them with particular advantages and shortcomings (Table 1).
| Composition | Type | Advantages | Disadvantages | Payload | Homing to | Ref. |
| Polymeric NP | Poly(D,L-lactide), poly(D,L-lactic acid-co-α,β-malic acid),Poly-L-lactic acid | Biocompatible, FDA approved, versatility, efficient upload by stem cells, human HSCs, MSCs retain differentiation potential | Biphasic and uncontrolled payload release | Antitumour drugs, | Glioma tumours | [77-79] |
| Silica NP | Mesoporous silica, amorphous silica | Fast uptake, negligible toxicity, long retention inside cells, lysosomal activation not associated with oxidative stress | Long term remanence within cells/tissues | Antitumour drug doxorubicin, fluorescent dye, paclitaxel | Mammary tumours, infarcted heart | [80-82,92] |
| Liposomal NP | Liposomes | Relatively facile manufacturing, versatility for drug delivery | Less-efficient uptake process, higher concentrations needed, which can be toxic to cells | 6-coumarin | Glioma tumours | [83] |
| Magnetic nanoparticles | Iron oxide NPs, magnetite, maghemite | Cellular tracking potential, reduced cell toxicity, high loading efficiency | Can induce oxidative stress in carrier cells | NGF, FGF | Dorsal root ganglia, HUVECs | [84-86] |
Poly (D, L-lactide) (PLA) and poly (D, L-lactic acid-co-α, β-malic acid) (PLMA) have the advantage of being already in clinical use and FDA approved, can be tuned in variable sizes (ranging from micrometres to several nanometres. Polymeric NPs are uploaded by cells by means of pinocytosis or clathrin-mediated endocytosis[75]. Particle upload is time and concentration dependent, with good loading efficiency, viability and phenotype preservation demonstrated in various cell types[76]. Polymeric NPs have a hydrophobic core with an important loading capability and a hydrophilic shell that provides stability and can be designed to encapsulate hydrophobic or hydrophilic molecules, as well as proteins and nucleic acid. After internalization by the cell, polymeric NPs such as PLA and polyglycolide (PLGA) are able to disrupt the lysosomal membrane, where they initially accumulate due to modifications in the surface charge, and escape into the cytoplasm[77]. While the majority of studies involving stem cell polymeric nanoparticle loaded drug delivery have aimed to develop anticancer therapies, the potential use for regenerative medicine purposes is promising[78]. Human HSCs and MSCs loaded with Poly-L-lactic acid (PLLA) and PLLA-Fe complexes were shown to retain viability and main phenotypic characteristics as well as differentiation potential, qualifying as a method for drug-enhanced cell therapy[79].
Negatively charged silica NPs are shown to be uptaken by clathrin-mediated endocytosis and to co-localize with the cell lysosomal system. Drug-loaded silica NPs were shown to be uploaded by decidual stem cells without affecting their viability and differentiation potential[80]. Human bone marrow MSCs loaded with amorphous silica NPs were shown to retain viability and differentiation and to engraft within the beating heart in a mouse model of cardiac ischaemia, offering a promising tool for cell tracking and potential drug delivery[81]. Mesoporous silica NPs conjugated with a fluorescent dye (cyanine) could be internalized by human MSCs, making possible the discrimination between viable and early apoptotic cells and pointing towards their potential role as cell-tracking agents in preclinical studies[82].
Liposomal NPs are uptaken by stem cells via endocytosis and release their cargo into the intracellular space after fusing with the endosomal membrane. Human MSCs loaded with lipid nano-capsules retained their viability and differentiation as well as migratory and targeting potential. Liposomal NPs can be manufactured to carry a large variety of small molecules. The loading process is, however, less efficient than in the case of polymeric nanoparticles. The higher concentration of liposomes required for efficient loading may interfere with cell viability, a process that is cell dependent[83].
Magnetic NPs (MNPs) have been used as such or in combination with other biomaterials for targeted delivery as well as controlled release of information molecules. MNPs incorporated into poly-l-lactic acid (PLLA) containing nerve growth factor (NGF) were shown to direct the extension of neurite outgrowth from the dorsal root ganglia (DRG) in vitro as a model for controlled axonal regeneration[84].
Fluorescently labelled heparin mesoporous silica MNPs were shown to being able to deliver bFGF to human umbilical endothelial vein cells (HUVECs) up to 6 d in culture conditions, suggesting their potential use as multimodal carriers[85]. Stem cells loaded with iron oxide MNPs can achieve various degrees of magnetization, being traceable in vivo using clinically approved methods such as magnetic resonance imaging (MRI). High-resolution tracking of MNP-loaded stem cells using MRI allows cellular imaging, which is an important advantage for therapeutic application as it allows observing the cell fate after implantation. MSCs loaded with ferucarbotran could be observed using MRI while differentiating to functional neurons, a potential method for cellular tracking in stem-cell-based therapies[86].
Depending on the particle type, the upload process can extend from hours to several days of NP cell co-incubation. When considering potential clinical applications, the cell culture time needs to be decreased to a maximum. NP-dependent parameters such as NP colloidal stability, tendency to aggregate, surface charge or cell characteristics (e.g., phagocytic or non-phagocytic cells, logarithmic growth phase in culture) are known to influence particle upload. Several methods to increase the efficiency of NP uptake have been proposed with variable efficiency depending on the cell and NP type. NP functionalization with antibodies against MSC surface antigens (receptor-mediated endocytosis) was shown to speed up the upload process in cultured cells[87]. Several transfection agents, commercially available poly-cationic polymer poly-L-lysine, protamine sulphate or lipofectamine are available for enhancing NP upload in therapeutic cells. Physical methods that produce a temporary disruption of the cell membrane, such as electroporation, pulsed ultrasonication or application of a magnetic field, the later applicable for MNPs (magnetoporation), have variable efficiency in increasing specific NP upload. However, such methods impose supplementary cell manipulation procedures and can affect the cell viability and phenotypic stability; therefore, their use needs to be carefully poised when designing clinical applications[88].
Attaching NP complexes to the cell membrane is another possibility to endow stem cells with therapeutic molecules. Cell surface engineering, a complex area in cell engineering, can rely on a large variety of procedures such as chemical modification based on native membrane functional molecules, metabolic or gene engineering of the cell membrane to express functional groups, adsorption, or insertion of hydrophobic coupling groups or ligand/receptor interaction at the membrane interface are currently exploited to attach NPs to various cell types[89]. Several reports exist regarding surface modifications that could allow the trafficking of NP-conjugated molecules using stem cells. Liposome-based non-covalent membrane modifications allow attaching a cargo to engineered binding sites on the cell surface. Liposome fusion was obtained by co-incubating MSCs with lipid vesicles containing ketones and oxyamine molecules. The liposomes underwent spontaneous membrane fusion to present the respective molecules from the cell surfaces serving as sites for chemo-selective ligation with oxyamine-conjugated molecules in vitro[90]. The majority of anchoring techniques aim to modify membrane characteristics for improved targeting capabilities (see above) and fewer NP delivery processes.
Release of therapeutic cargo from NP-equipped stem cells takes place mainly by passive diffusion or exocytosis. Release is dependent on the particle size, cell type and modality of cytoplasmic NP storage. Diffusion and exocytosis can take place in time intervals ranging from minutes to several hours, while release from the cellular endosomal compartment, an energy consuming process, can take several days[73]. Ideally, a combination of the two mechanisms would be needed in order to enable uniform particle release. Pulsed and non-uniform payload release can impose hazards due to sudden drug or particle accumulation, not only compromising the therapeutic effect but also introducing supplementary hazards. In an ischaemic rat model subjected to MNP-loaded MSC injection, magnetic targeting resulted in vascular embolism and an inhomogeneous distribution of loaded cells, which prevented the intended targeted cell therapy from translating into a functional benefit[91]. Ingenious platforms for externally controlled release were shown to enhance the targeted release of a drug payload from decidua stem cells. Ultrasound-responsive mesoporous silica nanoparticles internalized by human decidua cells were shown to deliver their cargo under clinically available ultrasound frequencies both in vitro and in vivo, a promising strategy that could potentially be extended to the delivery of regenerative molecules[92].
For NP complexes attached to the cell membrane, the release profile can be engineered dependently on the type of receptor used for its conjugation to the cell membrane. A proposed solution is the design of slow-release systems by means of surface engineering as cell membrane biotinylation to obtain “synthetic biotin-avidin”-based cargo coupling[93].
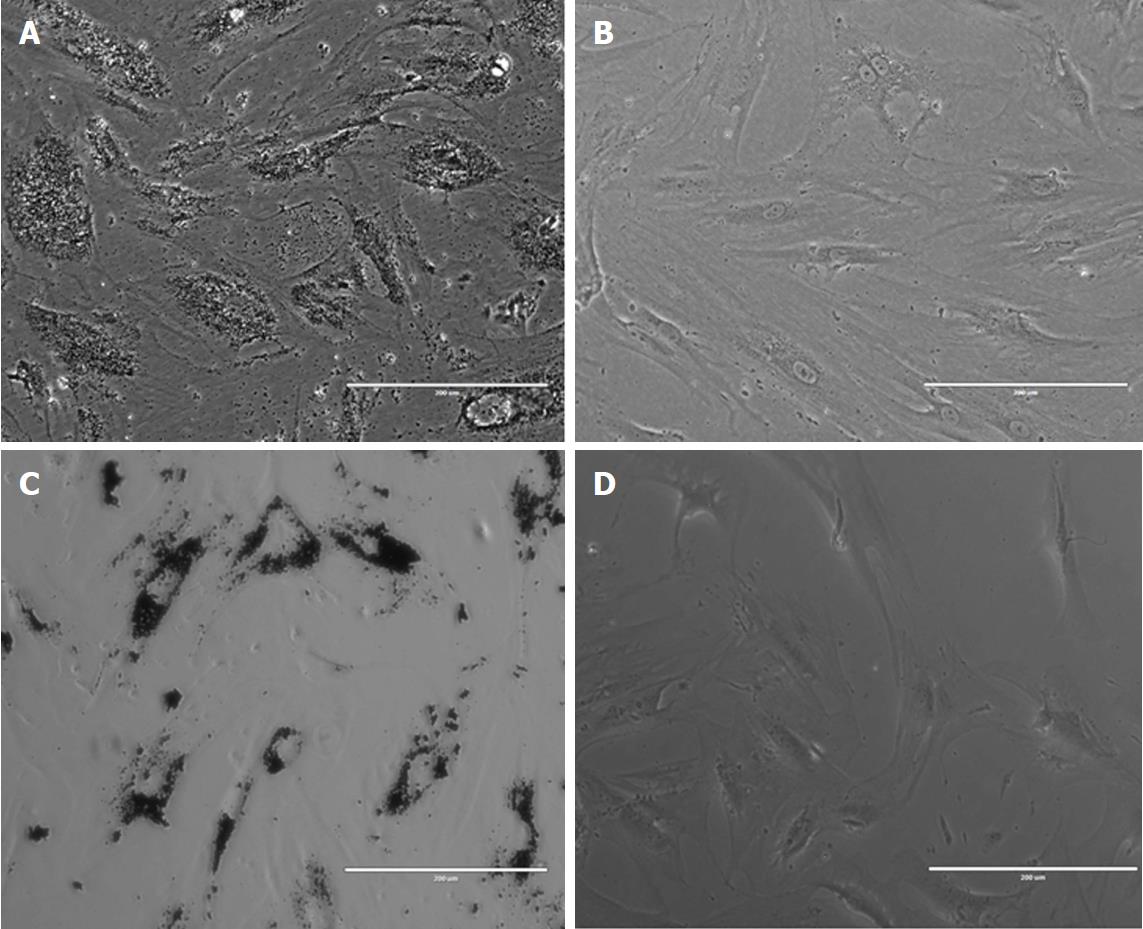
Due to their targeting capabilities, stem cells are sought as biological “carriers” for the delivery of therapeutic molecules. Many research groups are proposing stem-cell-mediated drug delivery for targeting tumour tissues or for cancer theranostic applications. The interest in combining the intrinsic role of stem cells with their potential use as drug carriers is increasing; however, the procedure is not yet a road much travelled. Even though the possibility of delivering a supplement of growth factors or immune modulating agents together with various cell populations for either cell and/or gene therapy or for tissue engineering is appealing, and several challenges need to be addressed. Issues that challenge regenerative medicine product development generally apply to the field of stem-cell-mediated drug delivery. Improved scalable methods for stem cell isolation, expansion and culture combined with the use of serum-free media formulations are mandatory for large-scale clinical applications. Innovative methods for stem cell characterization and automated sorting for cell-based product characterization are expected to provide improved quality control tools. Particularly for cell-based carriers, a basic requirement is to ascertain that cargo loading and presence not only is non-toxic to cells but also preserves their phenotypic features. Stem cell type, origin, and culture method as well as NP size, surface, electric charge, and coating determine the modality of the interaction, impact the cell biological potential. Every (stem) cell type and NP-drug cargo combination needs to be tested in this respect. With several exceptions (such as titanium wear debris), biocompatible NP internalization or surface conjugation has been shown to preserve MSC proliferative, differentiation and immunomodulation capabilities in different cell types[94] (Figure 3). Moreover, NPs can be engineered to increase their differentiation capability towards a desired lineage. Silver nanoparticles were shown to increase bone healing by increasing mouse MSC proliferation, chemo-attraction and osteogenic potential in a long bone fracture model[95]. Internalized MNPs were shown to increase MSCs osteogenesis via a long non-coding RNA INZEB2 mechanism[96]. Superparamagnetic iron oxide nanoparticles (SPIOs) used as contrast agents were shown to transiently and reversibly affect chondrogenesis in some cell types but not in others, stressing the importance of testing each particular cell type for a specific application[97]. Membrane structure and composition after particle internalization or conjugation needs to be assessed, as changes in surface antibodies could affect stem cell migratory capabilities. Interesting, various types of MNP internalization were shown to enhance the expression of chemokine receptor CXCR4 in MSCs and to improve their homing to a brain injury and glioblastoma model compared to the findings in non-loaded cells[98]. Stem cells loaded with MNPs present a particular interest in drug delivery, as they may be remotely actuated using external magnetic fields in order to increase delivery to target tissues and/or organs. Challenges regarding the intensity of the magnetic field, distance from the target organ (which influences the tracking capability), and risk of cell agglutination within the blood stream still need to be addressed. Magnetic force actuation of MNP-loaded stem cells has proved efficient for directed cell localization and consecutive repair of arterial injuries in small animal models or cartilage defects in larger animals (swine), paving the way for future clinical applications[99].
For NP drug complexes attached to the cell membrane, enzymatic or hydrolytic drug degradation within the biological environment is a serious challenge that increases side effects and lowers the therapeutic efficiency. Relevant in vivo models must be employed for testing collateral particle release within the blood stream and the specific pharmacodynamics of these particles, especially the tendency to accumulate within non-target tissues such as lung, spleen or liver. Drug-induced immunogenicity needs to be ruled out as well as potential interactions with cellular and non-cellular immune effectors within the biological milieu.
It should be noted that both cargo and NP loading can affect cell migratory and homing capabilities. Genetic engineering can be used in this case to modify cell surface receptors, to improve their migratory capabilities and to increase survival. The efficiency of such cell manipulation in every phase of cell homing needs to be tested in relevant models in vitro as well as in vivo, in combination with the drug release efficiency.
Stem cells are currently being tested by several groups as vehicles for targeting, imaging and treating tumours. For anticancer therapies, vehicle viability after successful cargo delivery is not an issue. In contrast, due to possible stem cell recruitment by tumours, it might prove safer to control cell proliferation and viability by means of cargo. Moreover, the demise of carrier cells triggers an immune response, contributing to tumour treatment[100]. For RM purposes, it would be beneficial not only to enable survival of these cells but also to improve their direct contribution to the regeneration process.
Controlling the stem cell fate by means of cargo could represent an appealing modality in steering regenerative processes. An MNP-based GF delivery and activation strategy was reported to be able to release TGF-β from its latent complex by application of an external magnetic field. Latent TGF-bioactive molecules such as GF, cytokines, DNA or miRNA could be delivered to control stem cell fate using MNPs. Magnetic core-shell MNPs composed of a highly magnetic core surrounded by a thin uniform gold shell enabled the delivery of controlling genetic materials [small interfering RNA (siRNA) and plasmid DNA (pDNA)] used to direct neural stem cell differentiation to neurons and oligodendrocytes. MNP-mediated RNA interference suppressed two key “neural switch” genes CAVEOLIN-1 and SOX9 that are responsible for oligodendrocyte and neuron differentiation, respectively. When conjugated to the surface of poly (ethylene glycol)-coated MNPs, molecules could be activated and released in a controlled manner[101]. Such a triggered GF-release strategy could be expanded to remote-control-delivered stem cell differentiation, increasing their participation in local healing.
Control of stem cell fate via the magnetic actuation of magnetic responsive cargo could be used to control the osteogenic differentiation of systemically delivered MSCs, preventing their adipogenic conversion for systemic osteoporosis treatment.
The large majority of current studies regarding stem-cell-mediated delivery of therapeutic agents are based on adult stem cells (MSCs or ESCs). MSCs and neural stem cells (NSCs) are promising targets for overcoming the blood-brain barrier to enable RNA and drug delivery for tumours or neurodegenerative disorders[102].
As is the case with any other stem-cell-based therapy, improved methods for cell collection and culture protocol standardization combined with innovative methods for donor and cell selection are expected to accelerate the transition into safe and efficient therapies. The stem-cell-mediated delivery of therapeutic agents for targeting regenerative sites poses special challenges. Cell procurement and culture that enables preservation and/or methods for augmenting the homing potential are needed for both native and genetically modified cells. The dynamic and phenotypic modification of NP-complex augmented cells needs to be described in detail as well as its effect in modifying the microenvironment of target tissues. Stem-cell-mediated delivery has the potential to consistently enhance the therapeutic effect of these cells for RM applications.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Saeki K S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Wobma H, Vunjak-Novakovic G. Tissue Engineering and Regenerative Medicine 2015: A Year in Review. Tissue Eng Part B Rev. 2016;22:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Badowski MS, Zhang T, Tsang TC, Harris DT. Chimeric antigen receptors for stem cell based immunotherapy. J Exp Ther Oncol. 2009;8:53-63. [PubMed] |
| 5. | Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 884] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 6. | Stoltz JF, de Isla N, Li YP, Bensoussan D, Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N. Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century. Stem Cells Int. 2015;2015:734731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 8. | Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 9. | Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 10. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 11. | Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 730] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 12. | Farber DB, Katsman D. Embryonic Stem Cell-Derived Microvesicles: Could They be Used for Retinal Regeneration? Adv Exp Med Biol. 2016;854:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 14. | Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (3)] |
| 16. | Khaldoyanidi S. Directing stem cell homing. Cell Stem Cell. 2008;2:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Miller FD, Kaplan DR. Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell. 2012;10:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 722] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 19. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1088] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 20. | Lämmermann T, Sixt M. Mechanical modes of 'amoeboid' cell migration. Curr Opin Cell Biol. 2009;21:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 21. | Yin Y, Li X, He XT, Wu RX, Sun HH, Chen FM. Leveraging Stem Cell Homing for Therapeutic Regeneration. J Dent Res. 2017;96:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Heissig B, Dhahri D, Eiamboonsert S, Salama Y, Shimazu H, Munakata S, Hattori K. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell Mol Life Sci. 2015;72:4759-4770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Barreca MM, Spinello W, Cavalieri V, Turturici G, Sconzo G, Kaur P, Tinnirello R, Asea AA, Geraci F. Extracellular Hsp70 Enhances Mesoangioblast Migration via an Autocrine Signaling Pathway. J Cell Physiol. 2017;232:1845-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 2012;6:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Hocking AM. The role of chemokines in mesenchymal stem cell homing to wounds. Adv Skin Wound Ca. 2015;4:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Stuermer EK, Lipenksy A, Thamm O, Neugebauer E, Schaefer N, Fuchs P, Bouillon B, Koenen P. The role of SDF-1 in homing of human adipose-derived stem cells. Wound Repair Regen. 2015;23:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Miller RJ, Banisadr G, Bhattacharyya BJ. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008;198:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Alexeev V, Donahue A, Uitto J, Igoucheva O. Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy. 2013;15:171-184.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, Ding X, Tian P, Tian X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal. 2013;2013:632972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 33. | Crowder SW, Horton LW, Lee SH, McClain CM, Hawkins OE, Palmer AM, Bae H, Richmond A, Sung HJ. Passage-dependent cancerous transformation of human mesenchymal stem cells under carcinogenic hypoxia. FASEB J. 2013;27:2788-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. 2014;5:e1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 37. | Cesarz Z, Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016;5:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 38. | Huang X, Zhang F, Wang Y, SunX , Choi KY, Liu D, Choi J, Shin T-H Cheon J, Niu G, Chen X. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS Nano. 2014;8:4403-4414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Fernandez-Pernas P, Rodríguez-Lesende I, de la Fuente A, Mateos J, Fuentes I, De Toro J, Blanco FJ, Arufe MC. CD105+-mesenchymal stem cells migrate into osteoarthritis joint: An animal model. PLoS One. 2017;12:e0188072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM, Lee MJ, Jang IH, Kwon SM, Lee JS, Kim JH. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832:2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Xinaris C, Morigi M, Benedetti V, Imberti B, Fabricio AS, Squarcina E, Benigni A, Gagliardini E, Remuzzi G. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22:423-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Jones GN, Moschidou D, Lay K, Abdulrazzak H, Vanleene M, Shefelbine SJ, Polak J, de Coppi P, Fisk NM, Guillot PV. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Transl Med. 2012;1:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Kim YS, Noh MY, Kim JY, Yu HJ, Kim KS, Kim SH, Koh SH. Direct GSK-3β inhibition enhances mesenchymal stromal cell migration by increasing expression of β-PIX and CXCR4. Mol Neurobiol. 2013;47:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Yu Q, Chen L, You Y, Zou C, Zhang Y, Liu Q, Cheng F. Erythropoietin combined with granulocyte colonystimulating factor enhances MMP-2expression in mesenchymal stem cells and promotes cell migration. Mol Med Rep. 2011;4:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Noiseux N, Borie M, Desnoyers A, Menaouar A, Stevens LM, Mansour S, Danalache BA, Roy DC, Jankowski M, Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361-5372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Tsai LK, Leng Y, Wang Z, Leeds P, Chuang DM. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology. 2010;35:2225-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the sdf-1/cxcr4 axis. BioMed Res Int. 2013;2013:561098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 48. | Nowakowski A, Walczak P, Lukomska B, and Janowski M. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cells Int. 2016;1:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627-5635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Hassmann-Poznańska E, Chodynicki S, Sulik M. [Tumor of the parapharyngeal space]. Wiad Lek. 1989;42:991-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 52. | Wang W, Xu X, Li Z, Lendlein A, Ma N. Genetic engineering of mesenchymal stem cells by non-viral gene delivery. Clin Hemorheol Microcirc. 2014;58:19-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Meacham JM, Durvasula K, Degertekin FL, Fedorov AG. Physical methods for intracellular delivery: practical aspects from laboratory use to industrial-scale processing. J Lab Autom. 2014;19:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Das J, Choi YJ, Yasuda H, Han JW, Park C, Song H, Bae H, Kim JH. Efficient delivery of C/EBP beta gene into human mesenchymal stem cells via polyethylenimine-coated gold nanoparticles enhances adipogenic differentiation. Sci Rep. 2016;6:33784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Watermann A, Brieger J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials (Basel). 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 56. | Coutu DL, Cuerquis J, El Ayoubi R, Forner KA, Roy R, François M, Griffith M, Lillicrap D, Yousefi AM, Blostein MD. Hierarchical scaffold design for mesenchymal stem cell-based gene therapy of hemophilia B. Biomaterials. 2011;32:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Penati R, Fumagalli F, Calbi V, Bernardo ME, Aiuti A. Gene therapy for lysosomal storage disorders: recent advances for metachromatic leukodystrophy and mucopolysaccaridosis I. J Inherit Metab Dis. 2017;40:543-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, Underwood RA, Song KM, Sussman M, Byers PH. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Myers TJ, Granero-Molto F, Longobardi L, Li T, Yan Y, Spagnoli A. Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther. 2010;10:1663-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Sack BK, Herzog RW, Terhorst C, Markusic DM. Development of gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev. 2014;1:14013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells. 2016;8:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 62. | Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 63. | Lee JM, Kim EA, Im GI. Healing of tibial and calvarial bone defect using Runx-2-transfected adipose stem cells. Tissue Eng Regen Med. 2015;12:107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, Ruggiu M, Olivera-Martinez I, Robert N, Lu Y. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564-3576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 65. | Pelled G, Snedeker JG, Ben-Arav A, Rigozzi S, Zilberman Y, Kimelman-Bleich N, Gazit Z, Müller R, Gazit D. Smad8/BMP2-engineered mesenchymal stem cells induce accelerated recovery of the biomechanical properties of the Achilles tendon. J Orthop Res. 2012;30:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Kraus TM, Imhoff FB, Reinert J. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord. 2016;17:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Yan J, Zhang C, Zhao Y, Cao C, Wu K, Zhao L, Zhang Y. Non-viral oligonucleotide antimiR-138 delivery to mesenchymal stem cell sheets and the effect on osteogenesis. Biomaterials. 2014;35:7734-7749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Wang M, Tan J, Wang Y, Meldrum KK, Dinarello CA, Meldrum DR. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc Natl Acad Sci USA. 2009;106:17499-17504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Yeh TS, Fang YH, Lu CH, Chiu SC, Yeh CL, Yen TC, Parfyonova Y, Hu YC. Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials. 2014;35:174-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Imagawa K, Hirato T, Ueda M. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol Ther Methods Clin Dev. 2017;6:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 71. | Xiong N, Zhang Z, Huang J, Chen C, Zhang Z, Jia M, Xiong J, Liu X, Wang F, Cao X. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 2011;18:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 73. | Park JS, Suryaprakash S, Lao YH, Leong KW. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. 2015;84:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 74. | Markides H, Rotherham M, El Haj AJ. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J Nanomater. 2012;5-6:11. [DOI] [Full Text] |
| 75. | Van Rijt S, Habibovic P. Enhancing regenerative approaches with nanoparticles. J R S Interface. 2017;14:20170093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2117] [Cited by in RCA: 2427] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 77. | Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, Montero-Menei C, Menei P. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393-8401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 78. | Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 751] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 79. | Brüstle I, Simmet T, Nienhaus GU, Landfester K, Mailänder V. Hematopoietic and mesenchymal stem cells: polymeric nanoparticle uptake and lineage differentiation. Beilstein J Nanotechnol. 2015;6:383-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Paris JL, de la Torre P, Manzano M, Cabañas MV, Flores AI, Vallet-Regí M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016;33:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Gallina C, Capelôa T, Saviozzi S, Accomaso L, Catalano F, Tullio F, Martra G, Penna C, Pagliaro P, Turinetto V. Human mesenchymal stem cells labelled with dye-loaded amorphous silica nanoparticles: long-term biosafety, stemness preservation and traceability in the beating heart. J Nanobiotechnology. 2015;13:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Accomasso L, Cibrario Rocchietti E, Raimondo S, Catalano F, Alberto G, Giannitti A, Minieri V, Turinetto V, Orlando L, Saviozzi S. Fluorescent silica nanoparticles improve optical imaging of stem cells allowing direct discrimination between live and early-stage apoptotic cells. Small. 2012;8:3192-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Ying X, Wang Y, Xu H, Li X, Yan H, Tang H, Wen C, Li Y. The construction of the multifunctional targeting ursolic acids liposomes and its apoptosis effects to C6 glioma stem cells. Oncotarget. 2017;8:64129-64142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Wu Q, Liu C, Fan L, Shi J, Liu Z, Li R, Sun L. Heparinized magnetic mesoporous silica nanoparticles as multifunctional growth factor delivery carriers. Nanotechnology. 2012;23:485703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Zuidema JM, Provenza C, Caliendo T, Dutz S, Gilbert RJ. Magnetic NGF-releasing PLLA/iron oxide nanoparticles direct extending neurites and preferentially guide neurites along aligned electrospun microfibers. ACS Chem Neurosci. 2015;6:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Lu CW, Hsiao JK, Liu HM, Wu CH. Characterization of an iron oxide nanoparticle labelling and MRI-based protocol for inducing human mesenchymal stem cells into neural-like cells. Sci Rep. 2017;7:3587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Sarkar D, Ankrum JA, Teo GS, Carman CV, Karp JM. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32:3053-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Lewis EE, Child HW, Hursthouse A, Stirling D, McCully M, Paterson D, Mullin M, Berry CC. The influence of particle size and static magnetic fields on the uptake of magnetic nanoparticles into three dimensional cell-seeded collagen gel cultures. J Biomed Mater Res B Appl Biomater. 2015;103:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Stephan MT, Irvine DJ. Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today. 2011;6:309-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 90. | Dutta D, Pulsipher A, Luo W, Mak H, Yousaf MN. Engineering cell surfaces via liposome fusion. Bioconjug Chem. 2011;22:2423-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Huang Z, Shen Y, Pei N, Sun A, Xu J, Song Y, Huang G, Sun X, Zhang S, Qin Q. The effect of nonuniform magnetic targeting of intracoronary-delivering mesenchymal stem cells on coronary embolisation. Biomaterials. 2013;34:9905-9916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Huang Z, Shen Y, Pei N, Sun A, Xu J, Song Y, Huang G, Sun X, Zhang S, Qin Q. The effect of nonuniform magnetic targeting of intracoronary-delivering mesenchymal stem cells on coronary embolisation. Biomaterials. 2013;34:9905-9916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Fliervoet LAL, Mastrobattista E. Drug delivery with living cells. Adv Drug Deliv Rev. 2016;106:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 94. | Farcal L, Torres Andón F, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E, Mech A, Hartmann NB, Rasmussen K, Riego-Sintes J. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One. 2015;10:e0127174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 95. | Zhang R, Lee P, Lui VC, Chen Y, Liu X, Lok CN, To M, Yeung KW, Wong KK. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine. 2015;11:1949-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 96. | Zhang W, Dong R, Diao S, Du J, Fan Z, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 97. | Saha S, Yang XB, Tanner S, Curran S, Wood D, Kirkham J. The effects of iron oxide incorporation on the chondrogenic potential of three human cell types. J Tissue Eng Regen M. 2013;7:461-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Riegler J, Liew A, Hynes SO, Ortega D, O'Brien T, Day RM, Richards T, Sharif F, Pankhurst QA, Lythgoe MF. Superparamagnetic iron oxide nanoparticle targeting of MSCs in vascular injury. Biomaterials. 2013;34:1987-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 99. | Ikuta Y, Kamei N, Ishikawa M, Adachi N, Ochi M. In Vivo Kinetics of Mesenchymal Stem Cells Transplanted into the Knee Joint in a Rat Model Using a Novel Magnetic Method of Localization. Cts Clin Transl Sci. 2015;8:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Hill BS, Pelagalli A, Passaro N, Zannetti A. Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget. 2017;8:73296-73311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 101. | Monsalve A, Rodriguez AC, Rinaldi C, Dobson J. Remotely Triggered Activation of TGF- With Magnetic Nanoparticles. IEEE Magn Lett. 2015;6:1-4. [DOI] [Full Text] |
| 102. | Aleynik A, Gernavage KM, Mourad YS. Stem cell delivery of therapies for brain disorders. Clin Transl Med. 2014;3:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |