Published online Dec 26, 2018. doi: 10.4252/wjsc.v10.i12.212
Peer-review started: August 9, 2018
First decision: August 31, 2018
Revised: October 18, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 26, 2018
Processing time: 137 Days and 22.5 Hours
To evaluate the angiogenic effect of platelet-rich plasma (PRP)-preconditioned adipose-derived stem cells (ADSCs) both in vitro and in a mouse ischemic hindlimb model.
ADSCs were divided based on culture medium: 2.5% PRP, 5% PRP, 7.5% PRP, and 10% PRP. Cell proliferation rate was analyzed using the MTS assay. The gene expression of CD31, vascular endothelial growth factor, hypoxia-inducible factors, and endothelial cell nitric oxide synthase was analyzed using reverse transcription polymerase chain reaction. Cell markers and structural changes were assessed through immunofluorescence staining and the tube formation assay. Subsequently, we studied the in vivo angiogenic capabilities of ADSCs by a mouse ischemic hindlimb model.
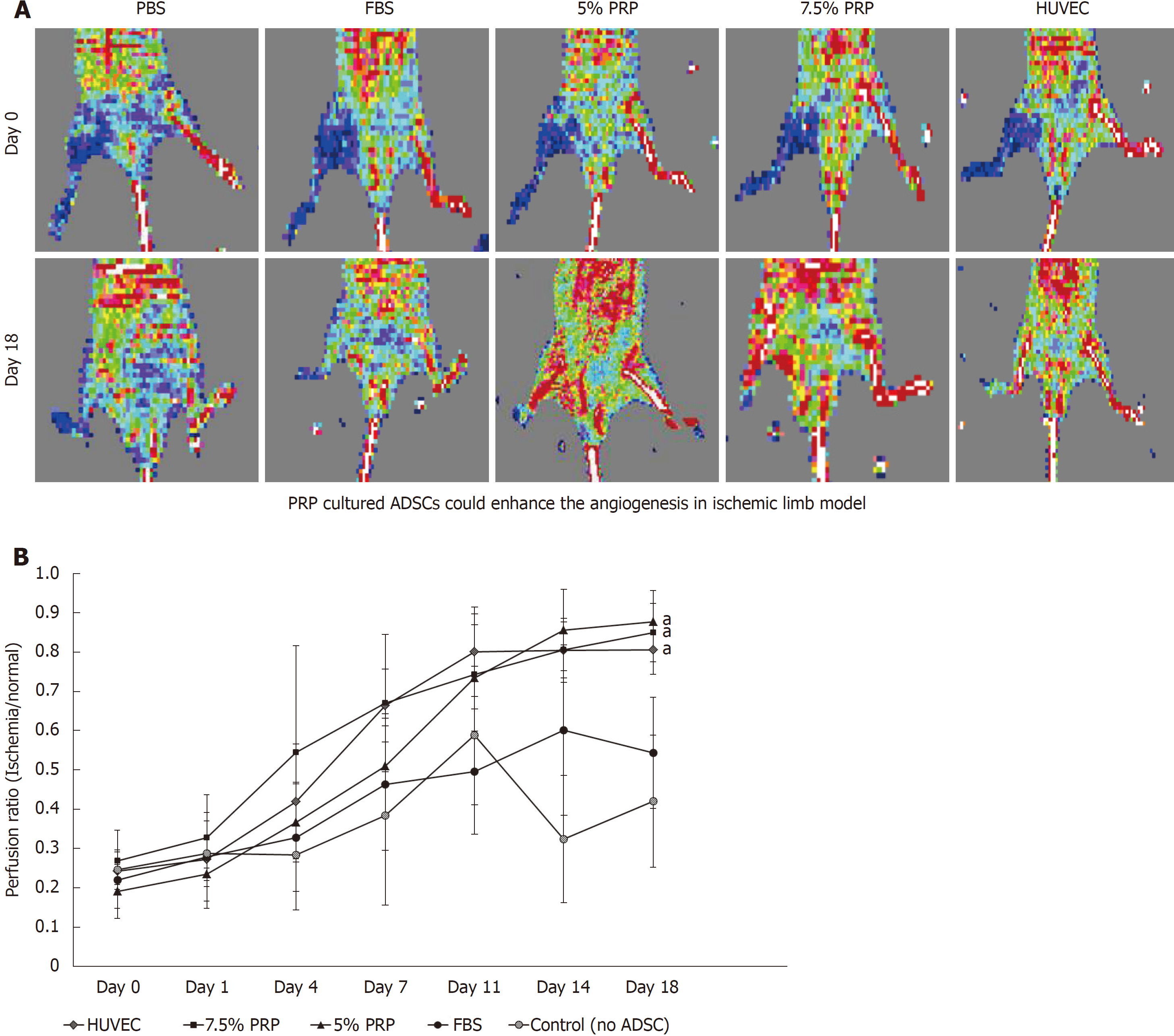
The proliferation rate of ADSCs was higher in the 2.5%, 5%, and 7.5% PRP groups. The expression of hypoxia-inducible factor, CD31, vascular endothelial growth factor, and endothelial cell nitric oxide synthase in the 5% and 7.5% PRP groups increased. The 5%, 7.5%, and 10% PRP groups showed higher abilities to promote both CD31 and vascular endothelial growth factor production and tubular structure formation in ADSCs. According to laser Doppler perfusion scan, the perfusion ratios of ischemic limb to normal limb were significantly higher in 5% PRP, 7.5% PRP, and human umbilical vein endothelial cells groups compared with the negative control and fetal bovine serum (FBS) groups (0.88 ± 0.08, 0.85 ± 0.07 and 0.81 ± 0.06 for 5%, 7.5% PRP and human umbilical vein endothelial cells compared with 0.42 ± 0.17 and 0.54 ± 0.14 for the negative control and FBS, P < 0.01).
PRP-preconditioned ADSCs presented endothelial cell characteristics in vitro and significantly improved neovascularization in ischemic hindlimbs. The optimal angiogenic effect occurred in 5% PRP- and 7.5% PRP-preconditioned ADSCs.
Core tip: We reported the in vitro angiogenic effect of platelet-rich plasma (PRP) treated adipose-derived stem cells (ADSCs) and the neovascularization ability of these cells in animal models. This is significant because we demonstrated that ADSCs presented endothelial cell characteristics after PRP treatment. We were the first to observe that treatment with PRP-preconditioned ADSCs significantly enhanced circulation in mouse ischemic hindlimbs models. Our result further showed that 5% and 7.5% PRP exerted the optimal effect on promoting angiogenesis of ADSCs and improving perfusion. We developed a stem cell-based, safe, and efficient way to promote peripheral circulation in animal model.
- Citation: Chen CF, Liao HT. Platelet-rich plasma enhances adipose-derived stem cell-mediated angiogenesis in a mouse ischemic hindlimb model. World J Stem Cells 2018; 10(12): 212-227
- URL: https://www.wjgnet.com/1948-0210/full/v10/i12/212.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i12.212
Peripheral artery disease (PAD) is caused by peripheral artery obstruction, which may lead to ischemic changes in the extremities. Due to insufficient blood flow in the musculature, patients may present with symptoms such as pain, claudication, or even tissue necrosis. Smoking, diabetes mellitus, hypercholesterolemia, hypertension, and renal insufficiency have all been reported to have high correlations with PAD. The pathological features of PAD include lumen obstruction caused by atherosclerotic plaques and destruction of vessel walls. Research has shown that elderly individuals and patients with diabetes mellitus are prone to these vasculature problems[1]. The current trend of the increasing populations of elder people and patients with diabetes mellitus is accompanied with the increased prevalence of PAD.
Current therapies for PAD are primarily aimed at relieving the discomfort and slowing the progress of the disease. In advanced PAD, revascularization surgery is indicated for large to medium-sized peripheral arteries with obstructions. However, ideal treatment for small arteries with obstructions has not been established. Therefore, treatments for obstructive lesions in small vessels are urgently required. Therapeutic angiogenesis provides a novel strategy for managing PAD; this strategy induces new vessel development in ischemic tissue, which can improve local perfusion.
Angiogenesis comprises many steps. Establishing stable and functional vascular networks is complicated. During ischemia, the damaged tissue releases growth factors to attract endothelial progenitor cells (EPCs). These cells proliferate, migrate, and form tubular structures, and finally achieve angiogenesis[2]. Microscopically, many growth factors are involved in angiogenesis. The angiogenic switch is initiated by hypoxia. Hypoxia-inducible factors (HIFs) are transcription factors that respond to hypoxia, and they play crucial roles in maintaining hemostasis during low oxygen conditions. During hypoxia, HIFs bind to targets, including the vascular endothelial growth factor (VEGF) gene, subsequently increasing the expression of downstream factors including transforming growth factor alpha and platelet-derived growth factor. Angiogenesis promotes endothelial cell proliferation and migration[3]. Endothelial cell nitric oxide synthase (eNOS), which is secreted by endothelial cells, exerts synergistic effects on neovascularization by increasing vessel wall permeability and promoting endothelial cell migration. CD31 also plays a crucial role in angiogenesis. It is a cell–cell adhesion molecule located on the endothelial cell membrane. Without CD31 stimulation, endothelial cells cannot form tubular structures. Through the synergistic effects of the mentioned factors, endothelial cells form new vessels at the ischemic site and subsequently establish a stable and functional perfusion system. Therefore, in research, these factors are commonly used as angiogenic markers for evaluating endothelial cell differentiation.
A previous study proved that mesenchymal stem cells (MSCs) can be used to form EPCs and promote angiogenesis, and MSCs are thus useful for vascular tissue engineering[4]. However, limited stem cell numbers circulate in the blood, which poses a major problem to the clinical application of these cells[5]. Although human adult stem cells can be obtained from many accessible sources, such as the bone marrow, teeth, and skeletal muscle, isolating human adult stem cells from the aforementioned tissue is difficult due to limited cell numbers and high donor site morbidities[6]. Recently, researchers have focused their attention on fat tissue-derived MSCs, adipose-derived stem cells (ADSCs). ADSCs were discovered in 2002 by researchers at University of California at Los Angeles; they have become a popular therapeutic strategy in current stem cell research. Abundant ADSCs can be retrieved from autologous fat tissue, and no controversy and ethical concerns are associated with these cells. In contrast to bone marrow-, teeth-, or skeletal muscle-derived stem cells, ADSCs are much easier to obtain. ADSCs can be collected through liposuction, which is a commonly performed cosmetic procedure[7,8]. Furthermore, after appropriate induction, ADSCs exhibit endothelial cell properties. All these characteristics render ADSCs more suitable for clinical use than other types of stem cells.
Fetal bovine serum (FBS) is widely used in research settings for the in vitro culture of ADSCs. However, culturing cells for therapeutic purposes in patients is associated with zoonotic disease transmission and xeno-immunization concerns. Alternative culture medium for ADSCs should be human-derived and should meet the criteria proposed by the International Society of Cellular Therapy (ISCT) and International Fat Applied Technology Society (IFATS)[9,10]. Because human serum is a natural reservoir of growth factors, and it has already been proved to be effective endothelial lineage differentiation media, several researchers have applied human serum products to culture ADSCs and induce angiogenesis in vitro. Recently, autologous conditioned serum, namely platelet-rich plasma (PRP), has shown great potential. PRP is an autologous reservoir of growth factors and cytokines. In summary, PRP has great potential to replace animal serum as culture medium.
To date, limited data are available on the effects of PRP on ADSCs. Our study evaluated the angiogenic potential of PRP-preconditioned ADSCs. In addition, ADSCs’ biological characteristics and their capability to induce angiogenesis both in vitro and in vivo were evaluated.
All experimental procedures were performed as per hospital regulations and medical ethics standards.
Concentrated human PRP (UltraGRO™) was purchased from AventaCell BioMedical Co., Ltd. (http://www.atcbiomed.com, United States). Blood donors were tested by the supplying company as per the United States regulations for the preparation of blood components. These donors were negative for mycoplasma, human immunodeficiency virus, hepatitis B virus, hepatitis C virus, human T-lymphotropic virus type 1 [determined by polymerase chain reaction (PCR) and serologic testing], anti-Trypanosoma cruzi antibody, and syphilis (determined by serologic testing).
Human raw lipoaspirates were derived from our patients undergoing selective suction-assisted lipectomy and were isolated following the procedure described by Zuk et al[11] with modifications. After harvesting, the lipoaspirates were washed extensively to remove blood cells, and the lipoaspirates, which were obtained under local anesthetics, were digested with 0.075% collagenase in a 37 °C water bath for 30 min. Subsequently, the cell pellet was collected through centrifugation and was incubated overnight in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% FBS at 37 °C in 5% CO2.
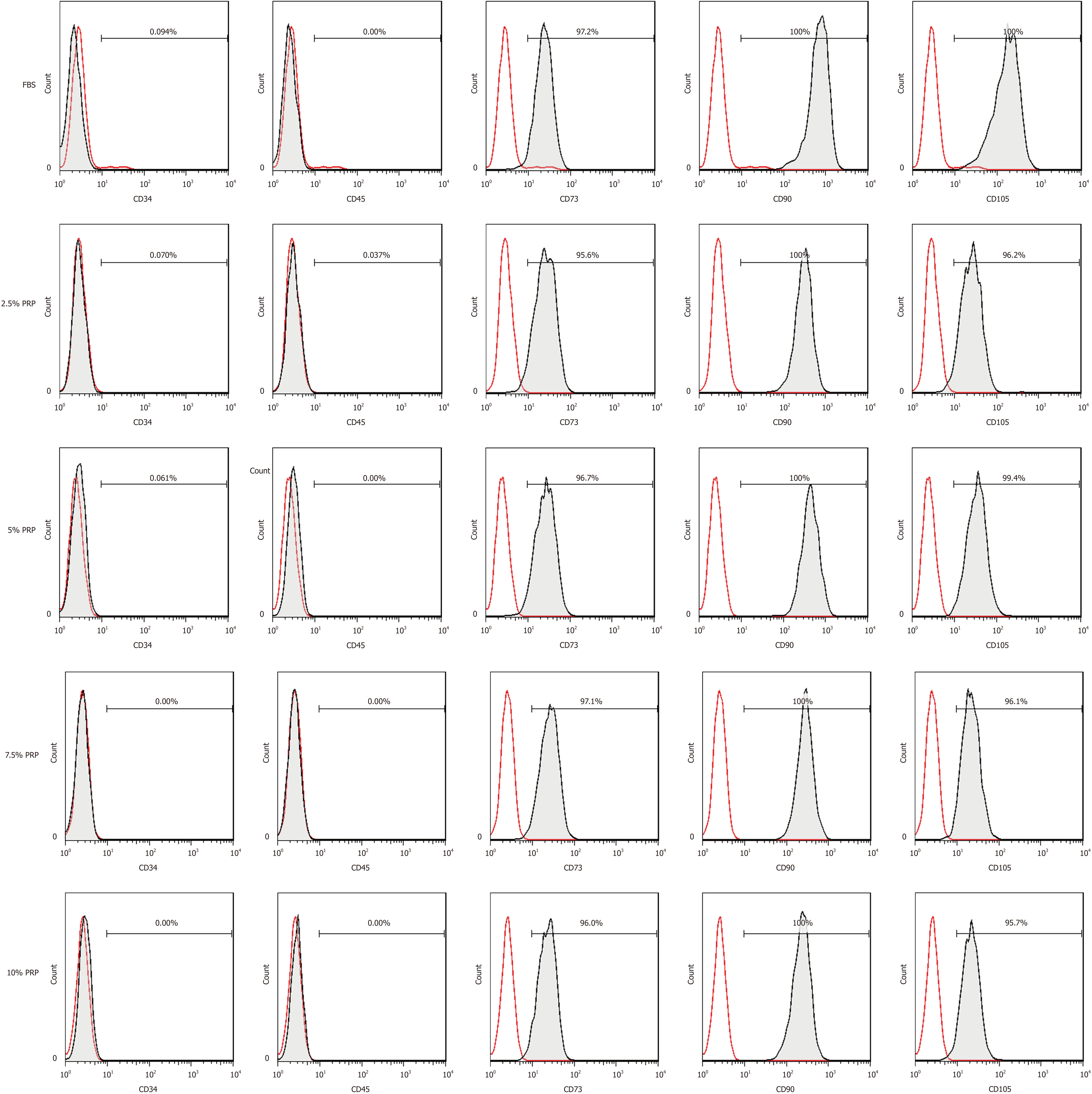
Flow cytometry analysis was performed to characterize the phenotypes of ADSCs. Cells were cultured in medium containing different concentrations of PRP (2.5%, 5%, 7.5%, and 10% PRP) or 10% FBS (control group). At least 1 × 106 cells per well were incubated with fluorescence-labeled monoclonal antibodies against human CD34 (BD Biosciences, San Jose, CA, United States), CD45 (BD Biosciences), CD73 (R&D Systems), CD90 (BD Biosciences), and CD105 (R&D Systems). After washing, the labeled cells were analyzed through flow cytometry using BD FACSCalibur™ and the BD CellQuest™ Pro.
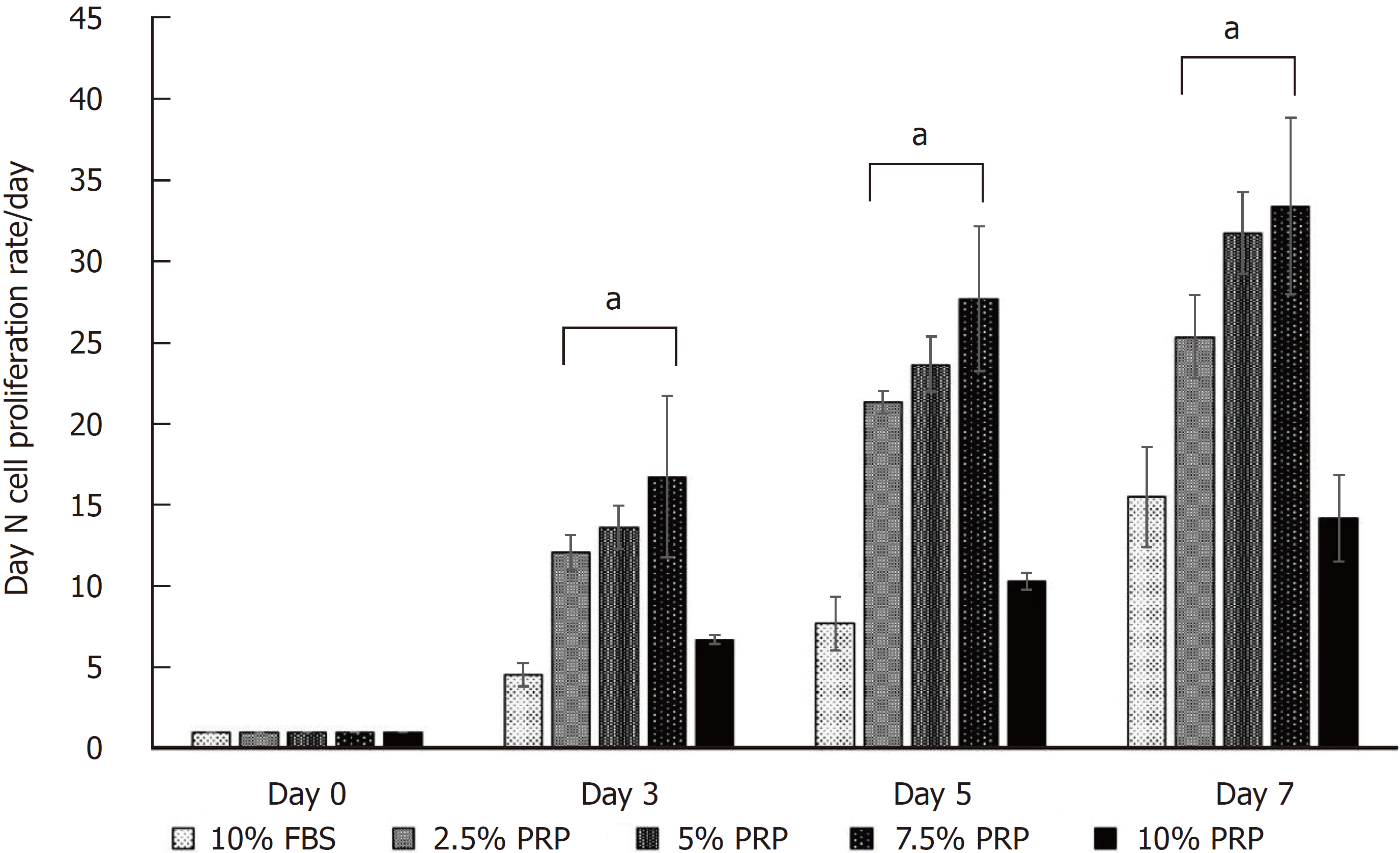
To determine the optimal concentration of PRP for ADSC proliferation, ADSCs were cultured in medium containing different concentrations of PRP (2.5%, 5%, 7.5%, and 10% PRP) or 10% FBS (control group). At least 5 × 103 ADSCs were seeded per well and were incubated at 37 °C in 5% CO2. Subsequently, the proliferation of ADSCs in each group was determined using the CellTiter 96 AQueous One Solution Reagent (Promega Co., Madison, WI, United States), which contains a novel tetrazolium salt (MTS). The tetrazolium salt MTS is reduced by living cells into a colored formazan product. The quantity of the formazan product is directly proportional to the number of viable cells. In our experiment, the colorimetric measurement of the formazan dye was performed at a wavelength of 490 nm on an enzyme-linked immuno sorbent assay plate reader (Molecular Devices, Sunnyvale, CA, United States). Cell numbers were determined using a calibration curve plotting the number of ADSCs vs the absorbance values on days 0, 3, 5, and 7.
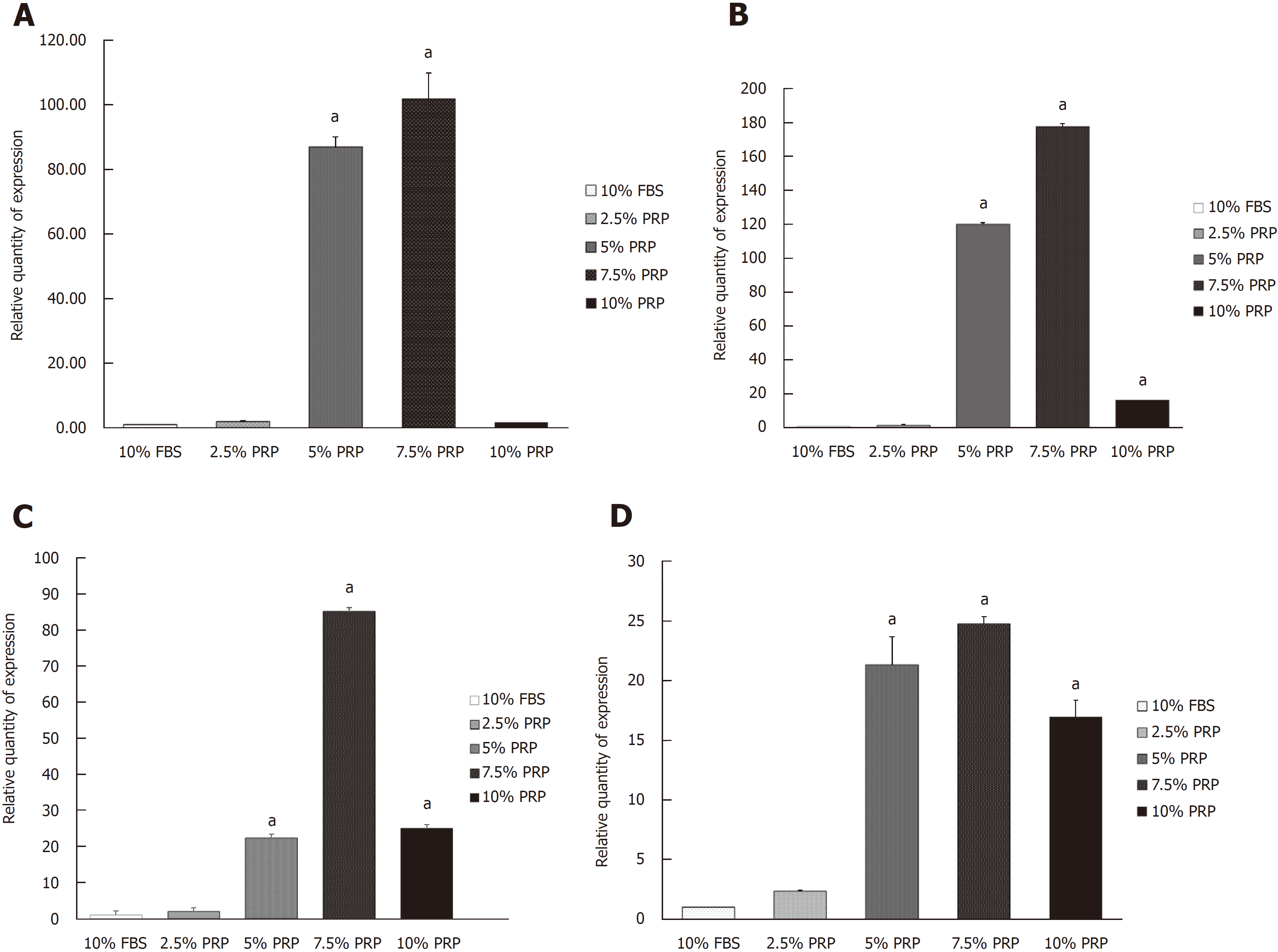
Mature and functional endothelial markers were evaluated through reverse transcription-PCR (RT-PCR). ADSCs were incubated in culture medium containing different concentrations of PRP (2.5%, 5%, 7.5%, and 10% PRP); 10% FBS was used as the control group. After culturing for 14 d, RNA was isolated using a GeneJET RNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, United States) in accordance with the manufacturer’s protocols. The expression of angiogenic- related genes, namely CD31 and CD34, was analyzed. The constitutively expressed gene encoding β-actin was used as an internal control in RT-PCR to normalize the amounts of mRNA in each sample.
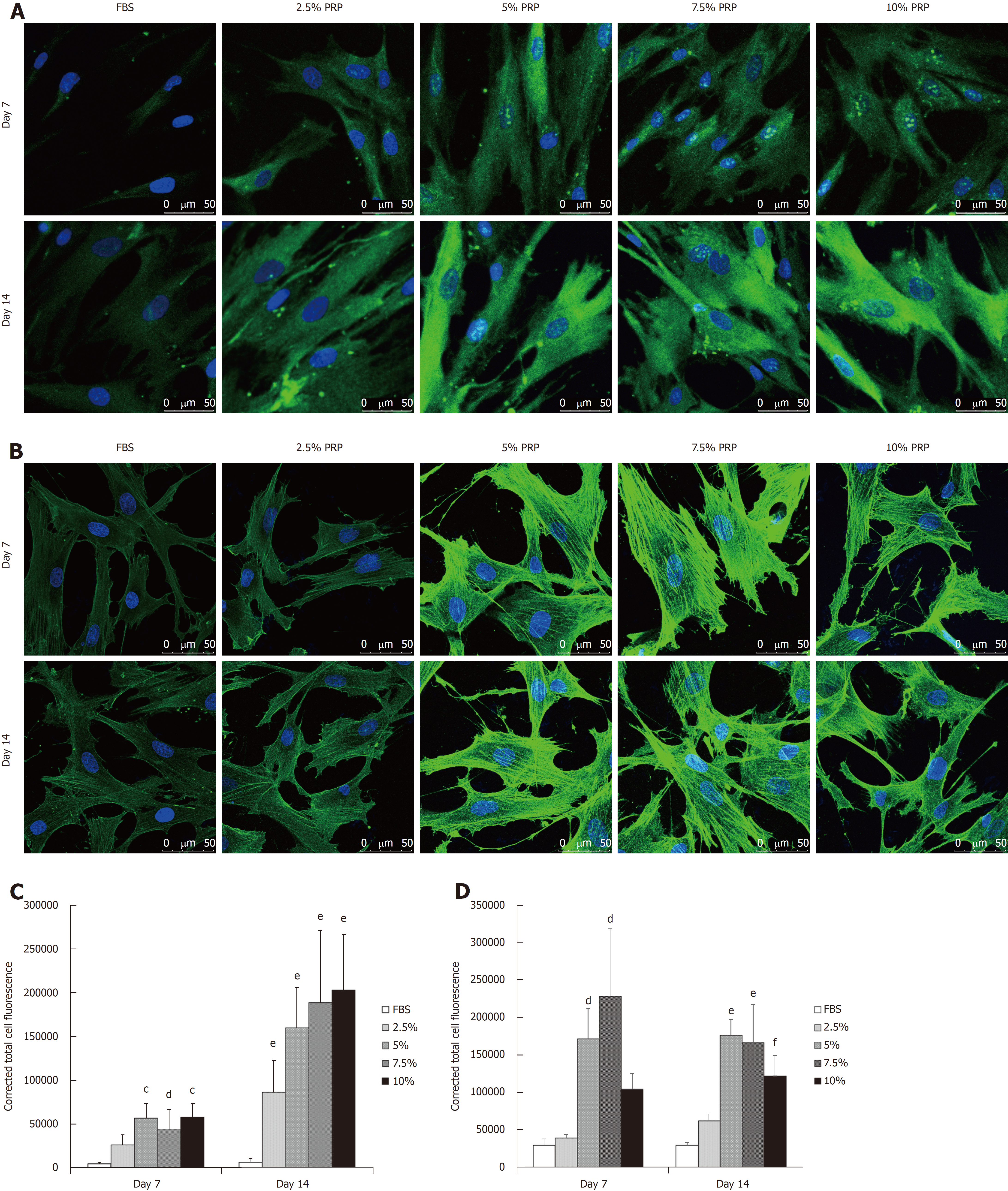
ADSCs were cultured in medium containing different concentrations of PRP (2.5%, 5%, 7.5%, and 10% PRP) or 10% FBS (control group). Approximately 1 × 104 cells were incubated at 37 °C in 5% CO2. On days 7 and 14, to identify the endothelial differentiation of ADSCs, immunofluorescence staining was performed using the following antibodies: rabbit polyclonal antibody (1:200) against human CD31 (1:50) and rabbit polyclonal antibody against VEGF. Antibodies with fluorescent labels were used. The immunostaining intensity was observed through confocal microscopy and was then quantified using Image J. The corrected total cellular fluorescence ratio was calculated using the following formula: corrected total cellular fluorescence = integrated density (area of selected cell × mean fluorescence of background readings).
Matrigel was thawed and suspended in 96-well plates and solidified at 37 °C for 30 min. ADSCs were cultured in medium containing different concentrations of PRP (2.5%, 5%, 7.5%, and 10% PRP) or 10% FBS (control group) for 14 d. Cells were then seeded at a density of approximately 1 × 105 cells per well and were labeled using Live-Dead Cell Staining Kits. The tubular structure was examined under a confocal microscope. Endothelial differentiation was assessed by determining the average length, total tube length, branch numbers, and total branch points through Image J.
Animal care and experiments were approved by the Local Ethics Committee for Animal Research Studies at Chang Gung Memorial Hospital. All nude mice were kept in laboratory conditions (controlled temperature 23 °C and humidity 50%) with free access to water and food for 4 wk prior to experimentation. ADSCs were preconditioned by culturing in medium containing 5% PRP, 7.5% PRP, and 10% FBS. Additionally, human umbilical vein endothelial cells (HUVECs) were used as a positive control. Mice administered with local injections of phosphate buffer solution (PBS) only (no ADSCs) were used as negative controls. General anesthesia was done by mixture of Zoletil and Xylazine 0.1 cc intraperitoneal injection before all experiments. The proximal portion of the right femoral artery of 8-wk-old nude mice was ligated using an electrosurgical pencil. About 3 × 106 preconditioned ADSCs and HUVECs were directly injected over the medial thigh muscle and lateral thigh muscle groups. Blood flow in both the ischemic hindlimb and normal hindlimb was measured using a laser Doppler blood flow meter on days 0, 1, 4, 7, 11, 14, and 18.
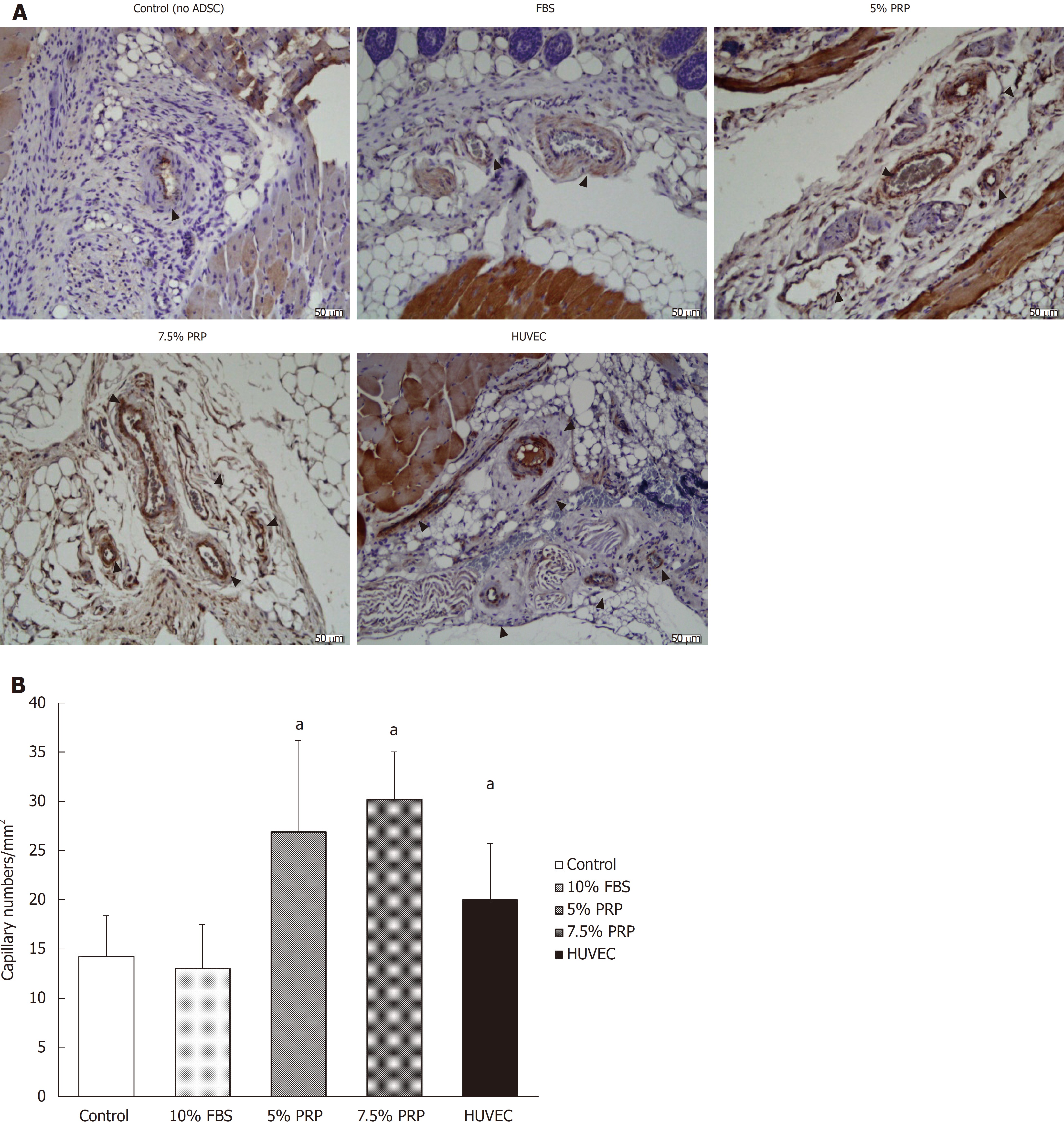
Nude mice were sacrificed on day 18. All mice were euthanized by carbon dioxide overdose for tissue collection. The muscle of the ischemic hindlimb was fixed in paraformaldehyde and sectioned into slices. To identify endothelial cells, immunohistochemical analysis was performed using rabbit polyclonal antibody against mouse CD31 antibody. The sections were then treated with diaminobenzidine and were stained using hematoxylin and eosin stain. Capillary density was determined by measuring the capillary numbers/mm2 under a microscope.
All the data are reported as mean ± standard deviation. Statistical analyses among the multiple group data are carried out using a one-way analysis of variance test to determine the significant differences. Turkey’s post-hoc test is used to determine the difference between any two groups with P < 0.05 considered statistically significant.
Flow cytometry was performed to characterize the phenotypes of ADSCs after their culture in medium containing PRP (Figure 1). Most of PRP- preconditioned ADSCs (98%-99%) were positive for endoglin receptor (CD105), the surface enzyme ecto-59-nucleotidase (CD73), and extracellular matrix protein (CD90). However, they were negative for markers of hematopoietic lineage (CD34) and the leukocyte common antigen (CD45). The cells presented cell markers specific to MSCs and these phenotypes meet the criteria proposed by the ISCT and IFATS for ADSCs.
The cell proliferation rate is presented as the growth ratio (%), which was calculated using the following formula: ADSC numbers on day 3, 5, or 7/ADSC numbers on day 0. The cell proliferation rate was increased from day 3 in PRP groups compared with the FBS group. At the endpoint (day 7), the proliferation rate of ADSCs was significantly higher in the 2.5%, 5%, and 7.5% PRP groups (25.348 ± 2.572, 31.778 ± 2.523, 33.400 ± 5.428 for 2.5% PRP, 5% PRP, 7.5% PRP, respectively) than in the 10% FBS (control group) and 10% PRP groups (15.483 ± 3.071 and 14.168 ± 2.650 for 10% FBS and 10% PRP; P < 0.01). The results suggested that 2.5%, 5%, and 7.5% PRP showed a higher ability to increase ADSC proliferation compared with FBS (Figure 2).
Angiogenic-related gene expression was analyzed by RT-PCR. Relative quantification of RT-PCR was done by calculating the difference of the dCt value between the target groups and the FBS group. Compared with the FBS group, the expression of HIF mRNA was significantly increased in the 5% and 7.5% PRP groups (P < 0.01; Figure 3A). The mRNA expression of endothelial cell markers, including CD31 and VEGF, was markedly higher in the 5%, 7.5%, and 10% PRP groups than in the 2.5% PRP and FBS groups (P < 0.01; Figure 3B and C). The eNOS mRNA expression level was also increased in the 5%, 7.5%, and 10% PRP groups (P < 0.01; Figure 3D). RT-PCR analysis revealed that the expression levels of HIF, CD31, VEGF, and eNOS genes were significantly higher in the 5% PRP and 7.5% PRP groups (P < 0.01). In RT-PCR, the expression of angiogenic-related genes was higher in the 5% and 7.5% PRP groups.
The protein production level was analyzed through immunofluorescence staining. After culturing for 14 d, CD31 expression was significantly higher in all PRP groups compared with the FBS group (P < 0.01; Figure 4A and C). According to the immunofluorescence staining results, the early elevated production of VEGF was noted from day 7 in the 5% and 7.5% PRP groups. On day 14, 5%, 7.5%, and 10% PRP groups showed considerable increases in VEGF production (Figure 4B and D). VEGF and CD31, which are produced by endothelial cells, are key proteins in angiogenesis. VEGF and CD31 production increased after ADSCs were cultured in medium containing PRP. The 5%, 7.5%, and 10% PRP groups showed a higher ability to promote both CD31 and VEGF production by ADSCs.
EPCs form tubes, connecting to each other, and are then arranged in clusters. Thus, morphological change is also a crucial parameter for evaluating the level of endothelial differentiation. Microscopy revealed the tubular structure of ADSCs in all PRP groups (Figure 5A). We quantified tube formation by determining the average length, tube numbers, and branch points (Figure 5B). Overall performance was evaluated based on the total tube length, which was calculated using the following formula: average length × tube numbers. Although the tubular structure was observed through microscopy, morphological changes in ADSCs cultured in 2.5% PRP were nonsignificant compared with those in the control group (P > 0.05). The result showed that ADSCs in the 5%, 7.5%, and 10% PRP groups showed more tube formation, more cell–cell interconnections (evaluated by branch points), and longer tubes (P < 0.01).
In our in vitro studies, 5% PRP and 7.5% PRP had higher abilities to promote the endothelial differentiation of ADSCs. We further designed an in vivo study to evaluate the angiogenic potential of these two groups. Blood vessel ligation was performed on the right hindlimb of nude mice. ADSCs from the PBS, FBS, 5% PRP, and 7.5% PRP groups and from HUVECs were applied to the wound immediately after surgery. Blood perfusion was measured immediately after surgery and on postoperative day 18 by using the laser Doppler blood flow meter. In representative images, red and blue indicated areas with normal blood perfusion and ischemia, respectively (Figure 6A). Image J was used to quantify laser Doppler images, and the result was expressed as the perfusion ratio (%), which was calculated using the following formula: blood flow in operated hindlimb/blood flow in non-operated hindlimb. Data revealed improved blood flow in the ischemic hindlimb after PRP treatment (Figure 6B). The revascularization rate remained low in the PBS and 10% FBS groups, with the ratios of 0.42 ± 0.16 and 0.54 ± 0.14, respectively, on day 18. The PRP and HUVEC groups showed significantly higher ratios on day 18 (0.88 ± 0.08, 0.85 ± 0.07, 0.81 ± 0.06 for 5% PRP, 7.5% PRP, and HUVECs, respectively) than the PBS group (0.42 ± 0.17; P < 0.01). No significant difference was observed in the perfusion ratios of the PRP and HUVEC groups (P > 0.05). Although serial laser Doppler images showed some natural recovery of hindlimb blood flow in control groups, administering PRP-preconditioned ADSCs to the ischemic site significantly increased tissue perfusion.
CD31 is a key glycoprotein expressed at endothelial cell intercellular junctions, and it is often used as an angiogenesis marker in research. In the present study, CD31 was used to demonstrate the presence of endothelial cells in histological tissue sections. Capillary density was assessed through quantification of CD31-positive capillaries, and the density was determined by measuring capillary numbers/mm2. Consistent with the findings obtained from laser Doppler image analysis, the capillary densities of the PRP groups were significantly higher than those of the control and FBS groups, as shown in Figure 7A and quantified in Figure 7B (26.95 ± 9.21, 30.22 ± 4.80, 13.03 ± 4.46, and 14.22 ± 4.14 for 5% PRP, 7.5% PRP, 10% FBS, and the control group, respectively). The 5% PRP and HUVEC groups had similar angiogenesis effects, and the 7.5% PRP group had the optimal result among all groups. The capillary density significantly increased in the 7.5% PRP group, even when compared with the positive control HUVEC group (30.22 ± 4.80 and 20.03 ± 5.67 for 7.5% PRP and HUVEC, respectively; P < 0.05). Overall, these data demonstrated that 5% PRP and 7.5% PRP-preconditioned ADSCs significantly enhanced physiologic neovascularization in ischemic tissue.
Our results showed that ADSCs cultured in medium containing PRP exhibited the properties of endothelial cells in terms of gene expression, angiogenic-related protein production, and morphological changes (i.e., tubular structure formation). The ischemic hindlimb model showed significantly improved blood perfusion after local injection with PRP- preconditioned ADSCs. Histochemical analysis of CD31 in muscle sections also provided the same result. Notably, compared with FBS, PRP exhibited a higher ability to promote the endothelial differentiation of ADSCs. The 5% PRP and 7.5% PRP groups had the optimal result among all groups. These findings suggest that 5% PRP and 7.5% PRP are suitable substitutes for FBS when culturing ADSCs and can achieve angiogenesis and improve perfusion in ischemic tissue.
Cell surface markers such as CD105, CD73, and CD90 are associated with the stemness of MSCs. Any change in cell surface markers indicates that stem cells may have committed to other linages. In our study, PRP was added to medium to culture ADSCs. However, some concerns still exist that PRP might change the stem cell characteristics of MSCs. Li et al[12] found that after culturing in PRP, MSCs maintained their stem cell marker expression as well as multilineage differentiation capacity. Moreover, another study found that the percentage of surface markers remained the same in ADSCs after culturing in PRP[13]. We showed similar findings in flow cytometry. Our ADSCs presented cell markers specific to stromal stem cells after PRP treatment.
PRP is a human blood derivative that is rich in growth factors. A previous paper reported that PRP could promote proliferation of ADSCs[14]. The ability of PRP to accelerate cell proliferation of stem cells may come from α-granules, which have many growth factors in a physiological ratio, such as platelet-derived growth factor, transforming growth factor-β, VEGF, epidermal growth factor, insulin-like growth factor, etc.[15]. These growth factors all play crucial roles for enhancing cell proliferation. However, only a few studies report the effects of different PRP concentrations on ADSC proliferation. Early studies found that PRP had a dose dependent effect on proliferation of ADSCs[16-18]. However, later studies had opposite opinions. One study demonstrated that a PRP concentration of 10% to 20% had the highest impact on cell proliferation. Proliferation rates declined with higher PRP concentration[19]. In another study, ADSCs were cultured in medium with PRP concentrations from 1% to 30%. The result showed that ADSCs were better grown in 5% and 10% PRP[20]. Another paper had a similar result that 10% PRP in the culture medium markedly promoted cell proliferation while a higher concentration (30%) of PRP had less effect on cell proliferation[21]. It is possible that an inhibitory effect is exerted if the concentration of growth factors or cytokines is too high. In our study, we noted that PRP significantly increased ADSC proliferation compared with 10% FBS based medium. However, the PRP concentration was not directly proportional to the proliferation rate. It is notable that 2.5%, 5%, and 7.5% PRP had better results compared with others. Kakudo et al[22] also observed a similar finding. The best dose of PRP for cell proliferation of ADSC may varies from study to study due to different ADSC and PRP preparation methods. It is difficult to compare results between studies due to the lack of standardized protocols for ADSC and PRP collection.
Platelets contain abundant α-granules. These α-granules play important roles in regulating secretory and angiogenesis pathways. However, α-granules not only release a variety of growth factors, but also contain regulatory proteins like the thrombospondin family. Thrombospondin-1 inhibits adhesion, proliferation, and tube formation of endothelial cells in culture, and it has been found to block neovascularization. Thrombospondin-2 also inhibits the migration and proliferation of endothelial cells. One study correlated large amounts of thrombospondin-1 detected in PRP with high concentrations of PRP could significantly decrease cell proliferation[23]. Whether the thrombospondin family is the most critical regulatory factor compromising angiogenic ability of PRP during high concentration needs further investigation.
In this study, markers including HIF, VEGF, CD31, and eNOS were used to evaluate ADSC-mediated angiogenesis. Gene expression was also confirmed through immunofluorescence staining. ADSCs cultured in PRP gained an endothelial phenotype, as demonstrated by the high expression of CD31 and VEGF. In a previous study, HIF was upregulated in ischemic tissues in response to low oxygen status[24]. Another study showed that through HIF-1 production, downstream genes were activated to achieve angiogenesis, cell proliferation, and cell survival[25]. ADSCs produced more HIF under low oxygen conditions[26]. The present study is the first to report that not only oxygen deprivation but also PRP treatment can assist ADSCs to produce more HIF, further activating angiogenesis.
HIF-1α is a subunit of HIF-1, and it can activate several angiogenic genes and their receptors under hypoxic status. VEGF is upregulated by HIF-1α, and it is the principal stimulatory factor in angiogenesis[27,28]. VEGF binds to VEGF receptor and activates the mitogen-activated protein kinase/extracellular signal–regulated kinase pathway to achieve cell proliferation. VEGF also activates the phosphatidylinositide 3-kinase/protein kinase B pathway to increase eNOS production[29]. eNOS then increases vessel wall permeability and facilitates the chemotactic migration of EPCs toward VEGF. VEGF also attracts cells including EPCs, mural cells, and hematopoietic stem cells to the ischemic site. These stem cells produce capillary plexuses and eventually form mature vessels. All these processes together cause the development of new vessels for vascular supply in ischemic limbs[30]. PRP is rich in VEGF and has been proven to enhance angiogenesis both in vitro and in vivo. ADSCs were also able to increase the VEGF level in the nude mouse model established through local injection, enabling vessel growth in ischemic tissue[31]. We hypothesized that local VEGF concentration could be further elevated through both work from PRP and ADSC. In our study, we proved that HIF and the downstream angiogenic-related genes VEGF and eNOS were upregulated in the PRP groups. High expression of the endothelial cell surface marker CD31 in the PRP groups was also strong evidence that PRP treatment induced the endothelial differentiation of ADSCs.
A variety of stem cells had been adopted to cell therapy. Regardless of their tissue origins, MSCs from different origins can express similar endothelial-relevant functions in vitro[32]. ADSCs are a kind of MSC, with the capacity to become various cells including adipocytes, chondrocytes, and osteocytes. ADSCs also retained the ability to differentiate toward an endothelial lineage. The elder age, cardiovascular disease, obesity, or tobacco use of donors does not alter the isolation. Besides endothelial cell related molecular expression, functional characteristics were evaluated by the tube formation assay. After proper differentiation, ADSCs formed tubular structures upon plating on Matrigel, indicating that ADSCs were able to differentiate toward endothelial cells and participate in angiogenesis. Our finding was consistent to previous studies that demonstrated that ADSCs participate in angiogenesis.
Single growth factor VEGF or boosting VEGF expression by curcumin had been applied in a tubular formation assay to promote capillary structure formation of stem cells[33,34]. However, stimulation of neovascularization involves complex steps and the result is influenced by multiple growth factors. Single growth factor VEGF use may have limitations to establish stable blood vessels. It is notable that PRP is a natural growth factor reservoir and had been proved to accelerate proliferation and stimulate capillary tube formation of endothelial cells[35]. Compared with FBS, PRP better supported the formation of lumen-like structures and the alignment of multicentric junctions of endothelial cells[36]. One study had found that formation of capillary like structures of HUVECs became maximal at 5% PRP[37]. Another study demonstrated that capillary-like structures were more prevalent in certain concentrations of PRP (0.5%, 1% and 3% PRP) compared with either the control group (0% PRP) or higher concentrations (5% and 10% PRP) in a co-culture system of HUVECs and human dermal fibroblast cells[38]. These two studies demonstrated PRP induced capillary–like structures of HUVECs in a bell-shaped dose-response curve. A previous study has proven that ADSCs differentiated into endothelial cells and produced capillary-like structures as HUVECs did in vitro[7]. However, no studies worked on the dose-dependent effect of PRP on ADSCs for formation of capillary-like structures. In our study, we found PRP significantly induced morphologic change of ADSCs. Further investigation indicated that tubular formation was proportional to PRP concentration between 2.5% to 7.5% PRP level, suggesting that proper ratio of angiogenic factors in PRP is important for formation of functional blood vessels by ADSCs.
From our results, the higher concentration of PRP lowered the proliferation rate of ADSCs and further reduced endothelial differentiation. Pro-angiogenic and anti-angiogenic molecules released by activation of plasma thrombin receptors during tissue injury gives an explanation to above phenomenon. As mentioned above, α-granules in the platelets contain regulatory proteins like the thrombospondin family. These proteins regulate angiogenesis by inhibiting MSC proliferation, tubular formation, and migration. Besides, the pro-angiogenic VEGF in α-granules and the anti-angiogenic endostatin in plasma were released upon platelet activation[39]. Balance between local VEGF and endostatin concentration accounts for the net biological effect on injured tissue[40]. High levels of anti-angiogenic molecules present in plasma should be considered when using high concentrations of PRP in tissue engineering.
Increasing research attention has been paid to PAD due to the increased disease prevalence. Various treatment strategies have been developed to induce new vessel formation and restore tissue perfusion through the use of exogenous molecular and cellular agents. The ischemic hindlimb model of mice has been widely used for the in vivo investigation of cell therapy.
Although preclinical results of single-dose growth factor use in ischemic animal models were promising, the therapy did not have long-lasting clinical effects due to the short half-life of growth factors[41]. Maintaining a stable level of growth factors is essential. Early studies have demonstrated that compared with the single-dose administration of VEGF, a drug delivery system that enabled the sustained release of growth factors significantly increased tissue blood flow, number of arterioles, and vascular density in the rabbit ischemic hindlimb model[42,43]. Gelatin hydrogels, polylactic-co-glycolic acid, and alginate are commonly used to make such granules. However, degradation time varies among different granules, and complete degradation may not be achieved. The residual foreign body is associated with a higher risk of infection and safety concerns, limiting the clinical application of granules. Gaining a comprehensive understanding of EPCs has enabled various stem cells to be utilized for endothelial differentiation and ischemic tissue repair. The focus has moved toward stem cell-based therapeutic angiogenesis, and ADSCs are promising stem cell types in therapeutic angiogenesis.
Many studies have demonstrated the angiogenic potential of ADSCs not only in vitro but also in vivo. In mouse ischemic hindlimb models, increased circulating endothelial cells were detected after local ADSC injection. The ischemic limbs recovered from muscle injury, and muscle sections exhibited increased vascular density[44,45]. Studies have found that neovascularization in ischemic tissue is attributed not only to the endothelial differentiation of ADSCs but also to their paracrine effects. Conditioned media obtained from ADSCs contained multiple angiogenic cytokines, including HIF, VEGF, fibroblast growth factor, and hepatocyte growth factor[46]. Through these growth factors, ADSCs augmented surrounding cell remodeling, reduced endothelial cell atrophy, and stimulated angiogenesis. However, the survival rates of ADSCs transplanted in the animal ischemic hindlimb model were variable. Multiple treatments have been developed to increase the survival and modify the angiogenic potential of ADSCs for cell therapy.
Under hypoxic conditions, the survival rate of ADSCs and the revascularization of animal ischemic hindlimbs are improved with the upregulation of HIF-1α and VEGF as the underlying mechanism[47,48]. Although researchers have reported that growth factors may have positive effects on the proliferation and endothelial differentiation of ADSCs, PRP treated ADSCs have never been applied in animal models. This study is the first to apply PRP, which is a natural reservoir of growth factors, to culture ADSCs and to evaluate cell angiogenic potential in vivo. Our hypothesis was that synergistic effects of multiple growth factors in PRP would increase the survival rate and endothelial differentiation of ADSCs. Application of these preconditioned ADSCs to ischemic tissue may achieve higher revascularization. PRP-preconditioned ADSCs had a higher ability to promote neovascularization in vivo compared with ADSCs preconditioned in common culture medium (i.e., FBS).
In the present study, we determined the effect of PRP concentration on cell angiogenic potential. HUVECs, which are known to have a strong ability to promote angiogenesis in animal ischemic hindlimb models, providing nearly 80% blood perfusion recovery (compared with the normal hindlimb) in our mouse experiment. However, 5% and 7.5% PRP-preconditioned ADSCs provided even more favorable results in the animal model. In the ischemic hindlimb, 5% and 7.5% PRP-preconditioned ADSCs achieved perfusion rates as high as 85% and 88%, respectively. The result of muscle histological sections staining was consistent with those obtained from laser Doppler image analysis.
In conclusion, our study provided a new therapeutic strategy for PAD. Both ADSCs and PRP offer many advantages, including abundant resources, efficient preparation, and safety. Moreover, the clinical application of ADSCs and PRP is associated with lower risks of xeno-immune responses and zoonotic disease transmission. Previous studies reported that PRP had limited effects on the recovery of blood perfusion and wound healing, which was mostly likely due to the rapid degradation of PRP in vivo. Therefore, instead of direct injection into tissue, ADSCs were preconditioned with PRP before cell implantation, which improved the angiogenic potential of cells and thus increased neovascularization in ischemic limbs. The balance between local angiogenic and proangiogenic factors accounts for the net biological effect on injured tissue. We further observed that 5% and 7.5% PRP provided the optimal effect on enhancing the angiogenic potential of ADSCs. Based on this finding, we believe that PRP treated ADSCs may be clinically applied for treating ischemic tissues and promoting wound healing in the future. Further research should focus on increasing the rate of ADSC differentiation into mature endothelial cells and finding key regulators for three-dimensional tubular structure formation in these cells. The optimal goal is to use ADSCs to form stable and functional vessels for patients with PAD.
Peripheral artery disease (PAD) is caused by peripheral artery obstruction, which may lead to ischemic changes in the extremities. In advanced PAD, revascularization surgery is indicated for large to medium-sized peripheral arteries with obstructions. However, ideal treatment for small arteries with obstructions has not been established until now.
Therapeutic angiogenesis provides a novel strategy for managing PAD. Mesenchymal stem cells can be used to promote tissue angiogenesis. Among all mesenchymal stem cells, adipose-derived stem cells (ADSCs) are plentiful, easy to retrieve with less donor site morbidity, and free from ethical concerns, making it a good candidate for therapeutic angiogenesis. Fetal bovine serum (FBS) is widely used in research settings for culturing ADSCs. However, culturing cells for therapeutic purposes in patients is associated with zoonotic disease transmission and xeno-immunization concerns. Platelet-rich plasma (PRP) is an autologous reservoir of growth factors and cytokines, which have great potential to replace animal serum as culture medium.
To date, limited data are available on the effects of PRP on ADSCs. Our study evaluated the angiogenic potential of PRP-preconditioned ADSCs. In addition, ADSCs’ biological characteristics and their capability to induce angiogenesis both in vitro and in vivo were evaluated.
ADSCs were divided based on culture medium: 2.5% PRP, 5% PRP, 7.5% PRP, 10% PRP, or FBS as control. In vitro, we studied the cell proliferation rate, endothelial cell specific genes expression and cell morphology change. In vivo, we studied the angiogenic capability of ADSCs by mouse ischemic hindlimb mode.
The proliferation rate of ADSCs was higher in the 2.5%, 5%, and 7.5% PRP groups. The expression of hypoxia-inducible factor, CD31, vascular endothelial growth factor, and endothelial cell nitric oxide synthase increased in the 5% and 7.5% PRP groups. The 5%, 7.5%, and 10% PRP groups showed higher abilities to promote both CD31 and vascular endothelial growth factor production and tubular structure formation in ADSCs. According to laser Doppler perfusion scan, the perfusion ratios of ischemic limb to normal limb were significantly higher in the 5% PRP, 7.5% PRP and human umbilical vein endothelial cell groups compared with the negative control and FBS groups.
Our results showed that PRP-preconditioned ADSCs had a better ability to present endothelial cell characteristics in vitro. After PRP treatment, ADSCs significantly improved blood perfusion in ischemic hindlimbs. Furthermore, 5% PRP- and 7.5% PRP-preconditioned ADSCs exert the optimal angiogenic effect both in intro and in vivo.
We were the first study to observe the angiogenic effect of PRP-preconditioned ADSCs on ischemic hindlimb models. We were also the first to discuss the correlation between PRP concentration and angiogenesis of ADSCs. Based on our results, we believe that PRP and ADSCs could be clinically applied for treating ischemic tissues and promoting wound healing in the future. Further research should focus on increasing the rate of ADSC differentiation into mature endothelial cells and finding key regulators for three-dimensional tubular structure formation in these cells. The optimal goal is to use ADSCs to form stable and functional vessels for patients with PAD.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Yamaguchi DT, Zaminy A S- Editor: Ma RY L- Editor: Filipodia E- Editor: Song H
| 1. | Zhou R, Zhu L, Fu S, Qian Y, Wang D, Wang C. Small Diameter Blood Vessels Bioengineered From Human Adipose-derived Stem Cells. Sci Rep. 2016;6:35422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51-60. [PubMed] |
| 4. | Fujita Y, Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev. 2017;120:25-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1264] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 9. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (2)] |
| 10. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12635] [Article Influence: 701.9] [Reference Citation Analysis (2)] |
| 11. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5011] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 12. | Li H, Usas A, Poddar M, Chen CW, Thompson S, Ahani B, Cummins J, Lavasani M, Huard J. Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness. PLoS One. 2013;8:e64923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Cervelli V, Scioli MG, Gentile P, Doldo E, Bonanno E, Spagnoli LG, Orlandi A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med. 2012;1:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Shen J, Gao Q, Zhang Y, He Y. Autologous plateletrich plasma promotes proliferation and chondrogenic differentiation of adiposederived stem cells. Mol Med Rep. 2015;11:1298-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Tavakolinejad S, Khosravi M, Mashkani B, Ebrahimzadeh Bideskan A, Sanjar Mossavi N, Parizadeh MR, Hamidi Alamdari D. The effect of human platelet-rich plasma on adipose-derived stem cell proliferation and osteogenic differentiation. Iran Biomed J. 2014;18:151-157. [PubMed] |
| 17. | Van Pham P, Bui KH, Ngo DQ, Vu NB, Truong NH, Phan NL, Le DM, Duong TD, Nguyen TD, Le VT. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013;4:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Xiong BJ, Tan QW, Chen YJ, Zhang Y, Zhang D, Tang SL, Zhang S, Lv Q. The Effects of Platelet-Rich Plasma and Adipose-Derived Stem Cells on Neovascularization and Fat Graft Survival. Aesthetic Plast Surg. 2018;42:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Felthaus O, Prantl L, Skaff-Schwarze M, Klein S, Anker A, Ranieri M, Kuehlmann B. Effects of different concentrations of Platelet-rich Plasma and Platelet-Poor Plasma on vitality and differentiation of autologous Adipose tissue-derived stem cells. Clin Hemorheol Microcirc. 2017;66:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS One. 2014;9:e104662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31:212-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Hsu CW, Yuan K, Tseng CC. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 871] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 25. | Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1249] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 26. | Andreeva ER, Lobanova MV, Udartseva OO, Buravkova LB. Response of Adipose Tissue-Derived Stromal Cells in Tissue-Related O2 Microenvironment to Short-Term Hypoxic Stress. Cells Tissues Organs. 2015;200:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015:549412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 411] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 28. | Kakudo N, Morimoto N, Ogawa T, Taketani S, Kusumoto K. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1α Activation. PLoS One. 2015;10:e0139890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1424] [Cited by in RCA: 1361] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 30. | Bir SC, Esaki J, Marui A, Yamahara K, Tsubota H, Ikeda T, Sakata R. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50:870-879.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Ii M, Horii M, Yokoyama A, Shoji T, Mifune Y, Kawamoto A, Asahi M, Asahara T. Synergistic effect of adipose-derived stem cell therapy and bone marrow progenitor recruitment in ischemic heart. Lab Invest. 2011;91:539-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, Li X, Yang SG, Han ZB, Han ZC. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 33. | Trojahn Kølle SF, Oliveri RS, Glovinski PV, Kirchhoff M, Mathiasen AB, Elberg JJ, Andersen PS, Drzewiecki KT, Fischer-Nielsen A. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | You J, Sun J, Ma T, Yang Z, Wang X, Zhang Z, Li J, Wang L, Ii M, Yang J. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Res Ther. 2017;8:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Bir SC, Esaki J, Marui A, Sakaguchi H, Kevil CG, Ikeda T, Komeda M, Tabata Y, Sakata R. Therapeutic treatment with sustained-release platelet-rich plasma restores blood perfusion by augmenting ischemia-induced angiogenesis and arteriogenesis in diabetic mice. J Vasc Res. 2011;48:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Li X, Hou J, Wu B, Chen T, Luo A. Effects of platelet-rich plasma and cell coculture on angiogenesis in human dental pulp stem cells and endothelial progenitor cells. J Endod. 2014;40:1810-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Kakudo N, Morimoto N, Kushida S, Ogawa T, Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol. 2014;47:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Kakudo N, Morimoto N, Ogawa T, Hihara M, Notodihardjo PV, Matsui M, Tabata Y, Kusumoto K. Angiogenic effect of platelet-rich plasma combined with gelatin hydrogel granules injected into murine subcutis. J Tissue Eng Regen Med. 2017;11:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, Schattner M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Etulain J, Mena HA, Negrotto S, Schattner M. Stimulation of PAR-1 or PAR-4 promotes similar pattern of VEGF and endostatin release and pro-angiogenic responses mediated by human platelets. Platelets. 2015;26:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao K, Li Z, Zhao Q, Liu Z, Wu H. Induction of angiogenesis by controlled delivery of vascular endothelial growth factor using nanoparticles. Cardiovasc Ther. 2013;31:e12-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Doi K, Ikeda T, Marui A, Kushibiki T, Arai Y, Hirose K, Soga Y, Iwakura A, Ueyama K, Yamahara K. Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessels. 2007;22:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 588] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 47. | Yu WY, Sun W, Yu DJ, Zhao TL, Wu LJ, Zhuang HR. Adipose-derived stem cells improve neovascularization in ischemic flaps in diabetic mellitus through HIF-1α/VEGF pathway. Eur Rev Med Pharmacol Sci. 2018;22:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1702] [Article Influence: 81.0] [Reference Citation Analysis (0)] |