Published online Nov 26, 2018. doi: 10.4252/wjsc.v10.i11.172
Peer-review started: July 19, 2018
First decision: August 24, 2018
Revised: September 10, 2018
Accepted: October 17, 2018
Article in press: October 17, 2018
Published online: November 26, 2018
Processing time: 129 Days and 23 Hours
Pancreatic ductal adenocarcinoma is one of the most aggressive solid tumours of the pancreas, characterised by a five-year survival rate less than 8%. Recent reports that pancreatic cancer stem cells (PCSCs) contribute to the tumorigenesis, progression, and chemoresistance of pancreatic cancer have prompted the investigation of new therapeutic approaches able to directly target PCSCs. In the present paper the non-cancer related drugs that have been proposed to target CSCs that could potentially combat pancreatic cancer are reviewed and evaluated. The role of some pathways and deregulated proteins in PCSCs as new therapeutic targets are also discussed with a focus on selected specific inhibitors. Finally, advances in the development of nanoparticles for targeting PCSCs and site-specific drug delivery are highlighted, and their limitations considered.
Core tip: Pancreatic cancer is characterised by remarkable resistance to treatment conferred by pancreatic cancer stem cells (PCSCs). Unfortunately, most conventional treatments are unable to eradicate tumours. Recent research has focused on characterising PCSCs to accelerate the development of novel therapeutic strategies. In the present paper, we shed light on promising new strategies such as using non-cancer drugs as anti-cancer therapeutics, targeting of deregulated pathways and proteins of PCSCs, and using nanoparticles for improved drug delivery.
- Citation: Di Carlo C, Brandi J, Cecconi D. Pancreatic cancer stem cells: Perspectives on potential therapeutic approaches of pancreatic ductal adenocarcinoma. World J Stem Cells 2018; 10(11): 172-182
- URL: https://www.wjgnet.com/1948-0210/full/v10/i11/172.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i11.172
Pancreatic cancer comprises many types of cancers, of which the most common is an infiltrating neoplasm named pancreatic ductal adenocarcinoma (PDAC)[1], which derives from the pancreatic ductal tree[2]. PDAC is almost always fatal, it is refractory to conventional treatments, and consequently has a documented five-year survival rate as low as 8%. The major driver genes participating in the whole process of disease development include KRAS, TP53, CDKN2A, and SMAD4. With a near 100% KRAS mutation frequency, PDAC is considered the most RAS-addicted of all cancers[3]. PDAC is also characterised by a dense tumour microenvironment, perineural and vascular local growth, and early distant metastases. In particular, it typically has a tendency to metastasise preferentially to the liver where soluble factors and extracellular vesicles deriving from the primary tumour contribute to form a supportive niche[4]. Patients seldom exhibit symptoms. Therefore, early diagnosis of the tumour is very difficult. Indeed, the majority of patients are diagnosed when metastatic events have occurred or during advanced-stage disease. For this reason, primary prevention such as avoiding smoking and having a fat-poor diet is important[5]. Currently, surgery coupled with chemo or radiation therapy is the main treatment approach although it doesn’t present satisfactory results[6]. Moreover, disease can persist or recur with local and distant metastases. Most patients subjected to resection of the tumour die from metastasis within five years[7]. Despite its low efficacy, gemcitabine (a pyrimidine analogue) was the first-choice chemotherapeutic strategy in advanced PDAC for many years[8]. It is effective in only 23.8% of PDAC cases[9] due to dense tumour stroma and scarce diffusion of drug and to subsequent development of gemcitabine chemoresistance[10].
Recently, understanding of pancreatic carcinogenesis has improved and some new therapeutic options have been suggested. For example, it has been demonstrated that FOLFIRINOX, a chemotherapy regimen made up of four drugs (folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin), or nab-paclitaxel plus gemcitabine provide a survival benefit over gemcitabine alone[11]. However, we are still far from a substantially better life expectancy for patients since these new therapeutic options increase the median survival by only a few months.
A growing body of evidence suggests that the drug resistance and metastasis of PDAC are mainly influenced by the presence of cancer stem cells (CSCs). In the present paper, we aim to summarise the current understanding of pancreatic cancer stem cells (PCSCs) and analyse and discuss therapeutic options for targeting PCSCs.
It has recently been demonstrated that CSCs play critical roles in resistance to anticancer treatment and are responsible for metastasis in several human malignancies, including PDAC[12]. CSCs are rare immortal tumour cells, which have the ability to self-renew, produce differentiated progeny, form tumours in mice, and form non-adherent spheroids called tumour-spheres in vitro[13,14]. CSCs are more resistant than non-CSCs to chemotherapy and radiotherapy treatments because they have higher expression levels of anti-apoptotic proteins, ABC transporters, and multidrug resistance genes[15]. These cells reside in a niche, a specific hypoxic/necrotic microenvironment that includes different cell types (each one possessing distinct metabolic properties), such as fibroblastic, immune, endothelial, and perivascular cells, as well as extracellular matrix components, cytokines, and growth factors. In this environment, CSCs protect and reprogramme their metabolism and respond to the metabolism of surrounding cells, increasing tumour growth and preserving phenotypic plasticity[14,16]. Induction and maintenance of CSC phenotypes are related to more than 20 different transcription factors, including NF-κB and the hypoxia inducible factors[13,17]. Moreover, CSCs adjust their metabolism to their microenvironment by acquiring intermediate metabolic phenotypes or shifting from oxidative phosphorylation (OXPHOS) to glycolysis/Warburg effect. CSCs are also characterised by a high autophagic flux, which is involved in resistance to microenvironment stresses, such as hypoxia, starvation, or anticancer treatment[18]. Thus, it has been supposed that autophagy plays a significant role in the resistance to CSCs related anticancer therapy[19].
Pancreatic CSCs, first described in 2007[20], represent less than 1% of all pancreatic cancer cells[21] and are responsible for PDAC tumour growth (initiation, progression, and recurrence), maintenance, metastasis, and chemoresistance. The origin of PCSCs remains unknown. The hypothesized sources are: Tissue stem cells or progenitor cells, stem cells derived from bone marrow, or dedifferentiated cells that result from genetic mutation[22]. PCSCs can be identified by markers, such as CD133, CD24, CD44, ESA/EpCAM (epithelial-specific antigen), c-Met, ALDH1, DclK1, CXCR4, and Lgr5. However, a universal signature is still lacking[13,23,24]. The main signalling pathways of PCSCs, which are essential for self-renewal, are the epithelial to mesenchymal transition (EMT) process, and resistance to conventional therapies include Wnt/β-catenin, Sonic Hedgehog (SHH), and Notch. In addition, other biological aspects, such as autophagy, forkhead box protein M1 (FoxM1), mammalian target of rapamycin (mTOR), Bmi-1, NODAL/ACTIVIN, NF-κB and PTEN pathways, have been shown to be implicated in PCSC activity.
Importantly, PCSCs co-exist with other cellular and non-cellular components that constitute the tumour microenvironment (including cancer-associated fibroblasts, pancreatic stellate cells, and tumour-associated macrophages). Understanding the relationship between PCSCs and all these components is extremely important to improve the knowledge of the PCSC biology[12]. Recently, it has been demonstrated that PCSCs are involved in highly dynamic cross-talk with the PDAC parenchymal cells[25] by a symbiotic relationship that underlies the initiation and maintenance of early PDAC infiltration and metastasis. In particular, the secretome of PCSCs paracrinically inhibits parental cell growth and autocrinically stimulates their own growth and vascularity, while the secretome of parental cells both paracrinically inhibits PCSC growth and autocrinically inhibits their own growth. It is clear that to make a substantial impact on pancreatic cancer, it is necessary to eradicate PCSCs with targeted therapeutics[26]. For this reason, a complete molecular characterisation of PCSC biology is fundamental. Recently, we have characterised the proteome[7] and the secretome[27] of Panc1 CSCs, demonstrating the functional role of fatty acid synthesis and mevalonate pathways in PCSC viability and identifying secreted proteins involved in cancer differentiation, invasion, and metastasis. Through a combined proteomics and metabolomics approach we also found that Panc1 CSCs, as compared to the parental Panc1 cells, have induced expression of proteins and metabolites involved in glycolysis, pyruvate-malate cycle, folate cycle, pentose phosphate pathway, and lipid metabolism, and reduced expression of proteins and metabolites involved in the Krebs cycle, spliceosome, and non-homologous end joining pathway[7].
Chemoresistance is the major obstacle to successful cancer treatment. Many drugs are not able to eliminate PDAC, which represents the primary reason for tumour recurrence and metastasis. PCSCs are very resistant and can survive conventional treatments interfering with the total eradication of a tumour[16,23]. The mechanisms involved in the chemoresistance of CSCs include the metabolic inactivation of the drug and efflux of the drug from the cells, as well as mutation or deregulation of the drug targets[28]. In particular, an altered drug transport activity, as an over-expression of aldehyde dehydrogenase and proteasome, and a decreased expression of the human equilibrative nucleoside transporters (ENTs) and human concentrative nucleoside transporters (CNTs), play a key role in the chemoresistance of PCSCs[23].
As previously reported, PCSCs reside in niches that are responsible for the protection of cancer cells, tumour growth, and phenotypic plasticity. Critical components for the ever-changing tumour microenvironment and for construction of CSCs niche are Wnt/RSPO (R-spondin), c-Jun N-terminal protein kinase (JNK), Nodal/Activin, Notch, or Hedgehog proteins[23]. This specific CSCs microenvironment has also been proposed to contribute to drug resistance.
Chemoresistance is also related to the EMT process, which has a fundamental role in invasive and metastatic behaviour in PDAC. EMT, in pancreatic cancer cells, is controlled by several transcription factors, such as Zeb1, which suppresses the adhesion molecule E-cadherin by repressing the miR-203 (an inhibitor of stemness) and the miR-200 family members (which regulate expression of stem cell factors)[29]. Accordingly, it has been demonstrated that the class I HDAC inhibitor mocetinostat interferes with Zeb1 function, represses EMT, and restores the drug sensitivity of PDAC cells[30]. In particular, EMT contributes to enhanced resistance to gemcitabine because it leads to an increase in cancer cells with reduced expression of nucleoside transporters (ENT and CNT) that are involved in drug uptake[31]. Finally, it has been hypothesised that quiescence protects PCSCs from chemotherapeutic treatment, which usually targets rapidly proliferating cells.
It is broadly accepted that development of anti-cancer drugs to target determinant pathways and proteins of PCSCs will improve chemotherapeutic outcomes[15]. Eradication of these CSCs should be able to stop tumour progression and reduce future tumour insurgences[26,32]. Potential strategies to target PCSCs are discussed in the next section.
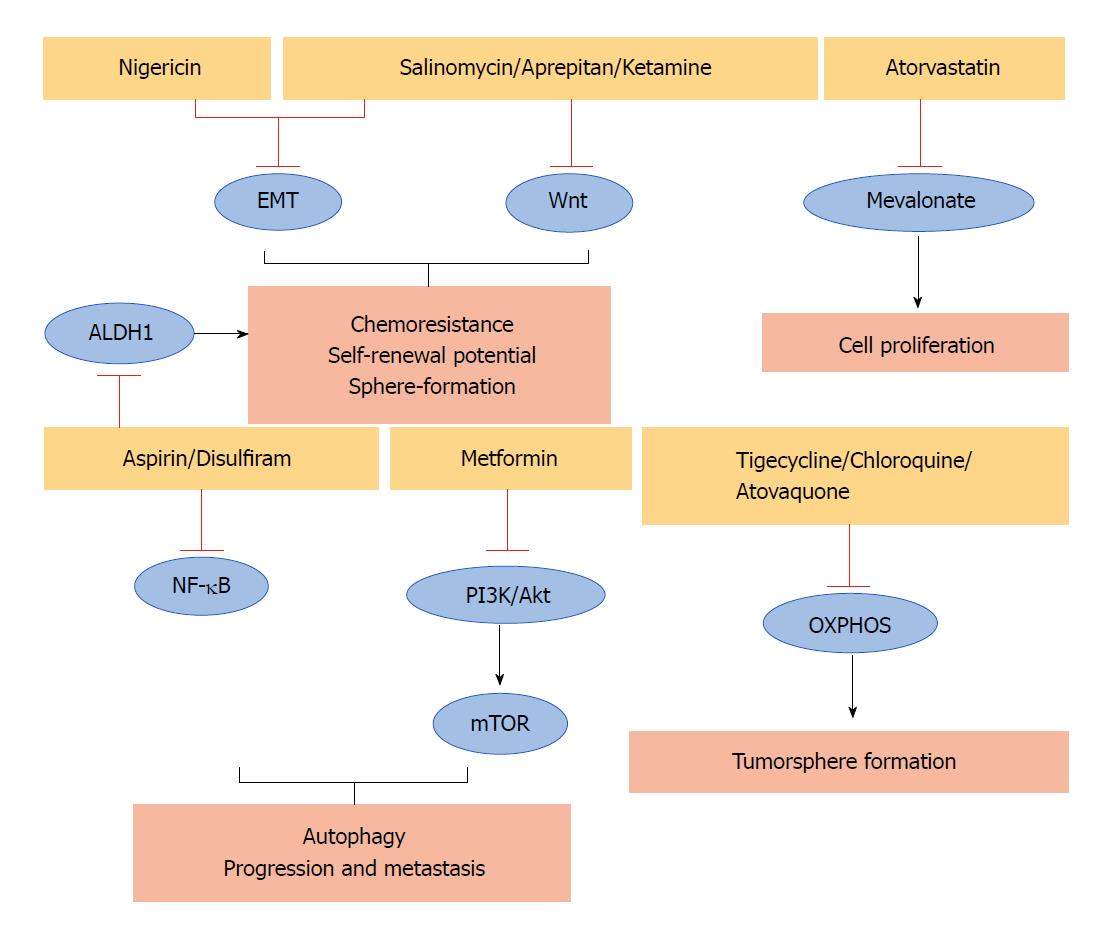
Some non-cancer related drugs that show anticancer effects against different human CSCs could also represent an option in PCSCs (Table 1). They act through different mechanisms of action including the inhibition of some important PCSCs pathways (Figure 1).
| Drug | Function | Relative pathway/process | Ref. |
| Salinomycin Azithromycin Nigericin Tigecycline | Anti-bacterial antibiotic | Wnt, EMT Mitochondria EMT OXPHOS | [33] |
| [35] | |||
| [38] | |||
| [29] | |||
| Chloroquine Atovaquone | Anti-malaria | OXPHOS OXPHOS | [39] |
| [36,40] | |||
| Aprepitant | Anti-emetic | Wnt | [41] |
| Ketamine | Anti-depressant | Wnt | [42,43] |
| Aspirin | Anti-pyretic Anti-inflammatory | ALDH1, NF-κB | [44,45] |
| Metformin | Anti-diabetic | mTOR, PI3K/Akt | [46-48] |
| Disulfiram | Anti-alcoholism | NF-κB | [49-53] |
| Atorvastatin | Anti-cholesterol | Mevalonate | [7,54] |
Antibiotics are among the molecules that exhibit extraordinarily diverse biological activities. For example, salinomycin, an antibacterial and coccidiostat ionophore drug, interferes with the activity of KRAS-4B, Wnt, and EMT pathways reducing the viability of breast CSCs[33]. Interestingly, it has also been demonstrated that salinomycin blocks tumour growth and the metastatic spread of PDAC in a genetically engineered mouse model[34]. In addition, the FDA-approved antibiotic azithromycin, which binds to the 50S subunit of the bacterial ribosome, inhibits tumour-sphere formation in PDAC and other cancers[35]. Also, the antibiotic tigecycline, developed in response to the antibiotic resistance of some bacteria, reduces the sphere formation of CSCs in pancreatic, breast, lung, and prostate cancers[29]. In particular, it eliminates the therapy-resistant chronic myeloid leukaemia CSCs[36], and a phase I clinical trial demonstrated the safety of its intravenous infusions in patients with acute myeloid leukaemia[37], supporting its transfer to clinical use. Moreover, it has been demonstrated that the antibiotic nigericin increases E-cadherin expression and inhibits the EMT process of CSCs leading to a reduction of invasion and metastasis of colorectal cancer[38]. This observation suggests that it should be further investigated to determine whether if it is also effective for targeting PCSCs.
Some anti-malarial agents may have the potential to target PCSCs. For example, it has been demonstrated that chloroquine has significant effects on PCSCs by inhibiting CXCR4 and Hedgehog pathways[39]. The same can also be said for another anti-malarial compound atovaquone, which acts as a potent and selective OXPHOS inhibitor, inhibiting the sphere-formation of CSCs in breast cancer[40].
Also showing promise for targeting PCSCs is aprepitant, an FDA-approved antiemetic drug that inhibits Wnt signalling, sphere formation, growth, and stemness of CSCs in colon cancer[41]. Ketamine, a drug used as an anaesthetic and depression, reduces CSCs traits and tumour growth in a colorectal cancer model. In particular, it acts by decreasing Wnt activity[42]. Notably, ketamine reportedly inhibits the proliferation of PDAC cells[43].
Salicylic acid, also known as aspirin, is another non-cancer related drug that may be a candidate for eliminating PCSCs in the successful treatment of PDAC. Indeed, aspirin, commonly used as an antipyretic and anti-inflammatory drug, counteracts PCSCs features such as ALDH1 activity, NF-κB signalling, self-renewal potential, and gemcitabine resistance[44]. A phase III trial confirmed the beneficial effect of aspirin as an adjuvant treatment to prevent disease recurrence and contribute to survival after primary therapy in breast, colorectal, gastro-oesophageal, and prostate tumours[45].
Metformin, a dimethylbiguanide used as an antidiabetic drug, is also able to counteract the features of PCSCs. It inhibits the mTOR and PI3K/Akt pathways, reducing the expression of PCSCs markers in pancreatic tissue, as well as the size and number of tumour spheres. Moreover, in vivo experiments demonstrated that metformin prevents progression and metastasis in PDAC[46]. Unfortunately, a phase II trial showed that metformin does not improve the outcome in patients with advanced metastatic PDAC treated with standard therapy[47,48]. These findings suggest that future research should include studies of more potent biguanides.
Another non-cancer related drug is disulfiram, a drug widely used to control alcoholism, which is involved in the inhibition of NF-κB, ERK and proteasome pathways in PDAC. It has been demonstrated that disulfiram in combination with chemotherapy or chemoradiation, is able to target PCSCs[49-52]. Notably, a phase IIb trial demonstrated that the addition of disulfiram to chemotherapy prolonged survival in patients with newly diagnosed non-small cell lung cancer[53].
Moreover, we have recently demonstrated[7] that atorvastatin, a drug used to lower blood cholesterol, reduces the viability of PCSCs. Accordingly, the anticancer effect of cholesterol-reducing agents has been demonstrated against other CSCs. To date, in a clinical setting statin intake was significantly associated with longer recurrence-free survival in hepatocellular carcinoma patients with hepatectomy[54].
Taken together, these findings indicate that repurposing established compounds to target PCSCs could represent a good strategy for combating PDAC. It is also economically advantageous and assures rapid translation into clinical because these compounds often are already approved by the FDA and show minor side effects compared to traditional chemotherapeutic drugs[29].
In the last ten years, many agents that target specific deranged pathways of pancreatic tumour cells have shown promise in preclinical studies. Accordingly, potential therapies targeting PCSCs could be developed based on their deregulated pathways and/or proteins (Table 2). As stated above, multiple signalling pathways are known to be important for stemness, including the Wnt/β-catenin, SHH, Notch, and mTOR pathways. Some compounds that inhibit the Wnt signalling pathway have been reported in the previous section on non-cancer related drugs (i.e., salinomycin, aprepitant, and ketamine). It has been demonstrated that crocetinic acid (a carotenoid obtained from saffron) is able to target PCSCs by inhibiting the expression of both SHH and smoothened proteins, which play a key role in the SHH pathways[55]. SHH and smoothened proteins lead to the activation of the Gli transcription factor and target genes involved in stem cell maintenance. In particular, crocetinic acid decreases the number and size of the spheroids in a dose-dependent manner and supresses the expression of DclK1, a PCSCs surface marker[55]. Another natural compound that inhibits the SHH pathways is sanguinarine (an isoquinoline alkaloid derived from Sanguinaria canadensis). It has been recently reported to be an effective agent for the inhibition of PCSCs[56]. It inhibits the self-renewal capacity of PCSCs, as well as their migration, invasion, and EMT by suppressing the SHH pathway. Recently, PCSCs have been efficiently eliminated by targeting the SHH pathway using the Gli inhibitor GANT61 in combination with rapamycin (an mTOR inhibitor)[57].
| Deregulated pathways | Compound or strategy | Ref. |
| Hedgehog | Crocetinic acid | [55] |
| Sanguinarine | [56] | |
| GANT61 | [57] | |
| Notch | RO4929097, shRNA | [58] |
| Quinomycin A | [59,97,98] | |
| mTOR | Rapamycin | [60] |
| AZD8055 | [61] | |
| Deregulated proteins | Compound or strategy | |
| FASN | Cerulenin | [7] |
| AnxA1 | siRNA | [64] |
| MARCKS | MANS peptide | [66] |
| Galectin-3 | Polysaccharide RN1 | [70] |
| PKM2 | Lapachol | [72] |
| Diallyl disulphide | [73] | |
| ERRγ | GSK5182 | [82,83] |
Another deregulated PCSCs pathway that can be targeted is Notch signalling. Its inhibition by γ-secretase inhibitor (RO4929097) as well as by Hes1 shRNA reduces the formation of tumour-spheres and the proportion of PCSCs[58]. Notch signalling can reportedly be inhibited by using quinomycin A (an antibiotic and also classifiable as a non-cancer related drug). Quinomycin A suppresses PCSCs by reducing Notch 1-4 receptors and by decreasing the expression of their ligands (Jagged1, Jagged2, DLL1, DLL3, and DLL4) of the downstream protein Hes1 and the γ-secretase complex[59]. Quinomycin A also decreases the expression of DclK1, CD44, CD24, and EPCAM, retarding the tumour-sphere formation of PCSCs[59]. Clinical trials published several decades ago and not related to pancreatic cancer indicated a modest activity of quinomycin A against some tumours.
Inhibition of mTOR signalling has also been proposed as a novel strategy for targeting CSCs. In particular, it has been shown that greater suppression of PCSCs is obtained by combining gemcitabine with the mTOR inhibitor rapamycin[60] or c-Met/RON inhibitor with the mTOR inhibitor AZD8055[61].
Changes in the expression of PCSC proteins may represent a good starting point to investigate potential therapeutic targets. Recently, we indicated that fatty acid synthase (FASN) might represent a means of eradicating PCSCs[7]. Treatment with cerulenin, a specific FASN inhibitor, led to a reduction of Panc1 CSCs viability and decreased the formation of spheroids. Accordingly, it has been demonstrated that FASN plays a pivotal role in the maintenance of stemness in other CSCs[62]. Among the potential PCSCs targets we identified annexin A1 (AnxA1)[7], which is an important player in the development and progression of different types of cancer, including pancreatic cancer, and plays a role in the maintenance of stemness and drug resistance in some CSCs[63]. Recent studies have shown that knockdown of AnxA1 decreases cell invasion and metastatic potential in several types of cancer, including PDAC[64].
Another potential therapeutic target that is both overexpressed and oversecreted by Panc1 CSCs and that should be investigated to reduce the viability of PCSCs is myristoylated alanine-rich C-kinase substrate (MARCKS)[7,27], a protein involved in cell motility, cell shape, cell cycle regulation, secretion, and transmembrane transport[65]. It has been demonstrated that a peptide (MANS peptide) that inhibits the function of MARCKS reduces lung cancer metastasis[66].
Another protein overexpressed and oversecreted in Panc1 CSCs is galectin-3 (Gal3)[7,27], which activates RAS signalling[67]. Many studies reported that Gal3 is implicated in cancer stemness, in particular by activation of Notch signalling[68], and that it may therefore represent a good therapeutic target[69]. Accordingly, it has been shown that down-regulation of Gal3 by an allosteric inhibitor, i.e., the polysaccharide RN1 (purified from the flower of Panax notoginseng), increases metastatic cancer cell apoptosis and decreases pancreatic cancer cell growth[70,71].
Another potential target of PCSCs we identified was pyruvate kinase isozyme M1/M2 (PKM1/PKM2)[7]. Although PKM2 is a key mediator of glycolysis in cancer cells, research focused on exploiting metabolic pathways for cancer therapy is still scarce. To date, anti-tumour effects have been demonstrated in melanoma cells following treatment with lapachol (a specific PKM2 inhibitor)[72], and inhibition of stemness has been reported in breast CSCs treated with diallyl disulphide which targets PKM2 (and also CD44 and AMPK signalling)[73]. Recently a new series of small molecule PKM2 inhibitors able to inhibit the growth of tumour cells has been synthesised[74]. It could be worthwhile to evaluate their efficacy also on PCSCs.
Another protein involved in metabolism that is overexpressed and oversecreted by Panc1 CSCs is lactate dehydrogenase A (LHDA)[7,27]. LDHA is an important supporter of glucose metabolism in cancer cells. It generates adequate extracellular lactate to provide a favourable microenvironment for CSCs growth and invasion[75]. Although inhibition of LDHA activity has been proposed as an approach to cancer therapy[76], a limited number of LDHA inhibitors are reported in the literature[77]. However, LDHA is transcriptionally regulated by the oncogenic transcription factor FoxM1[78], and some FoxM1 inhibitors (such as thiostrepton, troglitazone, and the FDI-6 molecule) have been reported that could indirectly lead to a reduction of LDHA[79].
Among the upstream regulators of deranged PCSCs proteins, we found there is the oestrogen-related receptor gamma (ERRγ)[7], which promotes metabolic reprogramming in CSCs, pluripotency, OXPHOS, and the glycolysis pathway[80]. A novel strategy for targeting PCSCs could be represented by the inverse agonists of ERRγ, which decrease OXPHOS and mitochondrial activity and promote apoptosis[81]. Accordingly, it has been demonstrated that GSK5182 (an inverse agonist of ERRγ) determines the up-regulation of p21 and p27, promotes G1 phase arrest, and leads to ROS accumulation and pluripotency inhibition in iPS cells[82-84].
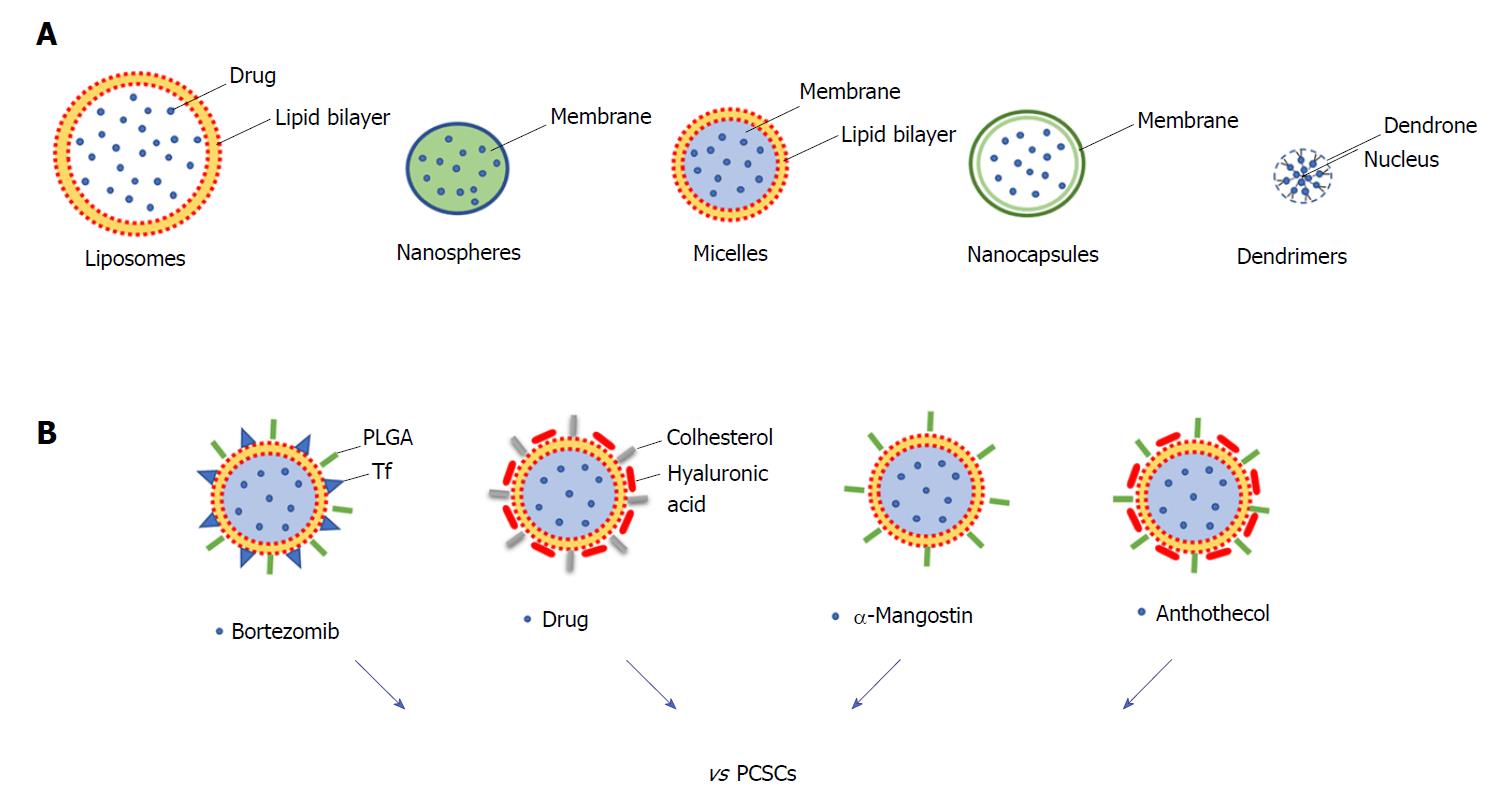
Surgery, chemotherapy, and radiotherapy are the most common anti-cancer therapeutic approaches; however, the non-specific targeting of cancer cells has made these approaches often non-effective with the consequence that higher doses of drugs need to be administered to reach the tumour region[85]. In order to improve the delivery of the drug, nanoparticles (NPs) have been developed to specifically and effectively target CSCs, reducing cytotoxicity and increasing the efficacy of treatments[86]. The different types of NPs include polymeric, magnetic, gold, and mesoporous silica NPs, and they provide a wide range of applications such as cancer therapy, tumour destruction through heating (hyperthermia), and drug/gene delivery[87,88]. In particular, for targeted drug delivery NPs comprise materials such as liposomes (100-400 nm), nanospheres (1-100 nm), micelles (10-100 nm), nanocapsules (10-1000 nm) and dendrimers (3-20 nm) (Figure 2A). These nanocarriers enhance the solubility and formulation of hydrophobic or water-insoluble drugs and control the drug delivery at the cancer tissue.
Some NPs have been developed to target pancreatic cancer, and liposomal formulations have gained regulatory approval[89]. The first clinical trial of NPs conducted in PDAC patients was done using a PEGylated colloidal gold-rhTNF nanomedicin, termed CYT-6091, which demonstrated that NPs greatly reduce the toxicity of chemotherapeutics and may target tumours[88]. In particular, some NPs have been developed to specifically target PCSCs (Figure 2B). PDAC is characterised by dense stroma with a high amount of hyaluronic acid (HA), which reduces drug delivery and interacts with CD44 surface marker regulating the invasion of PDAC cells. HA-based nanogel-drug conjugates with enhanced anticancer activity have been designed for the targeting of CD44-positive and drug-resistant tumours. These conjugates are based on membranotropic cholesteryl-HA (CHA) with various encapsulated drugs, such as the non-cancer related drug salinomycin, etoposide (a chemotherapeutic agent), or curcumin (a natural compound), and all have higher cytotoxicity in CD44-expressing drug-resistant PDAC cells compared to free drugs and to non-modified HA-drug conjugates[90]. Recently, HA-modified poly (dl-lactic-co-glycolic acid)-poly (ethylene glycol) (HA-PLGA-PEG) NPs have been developed for targeted delivery of TTQ (thio-tetrazolyl analogue of a clinical candidate, IC87114) to CD44 over-expressing cancer cells. In vitro results showed that cellular uptake led to higher cytotoxicity and enhanced intracellular accumulation of these NPs in high expressing CD44 MiaPaCa2 cells[91].
Natural product-based compounds can be an attractive strategy for the treatment of pancreatic cancer and could be integrated with NP approaches. For some of these, an inhibiting action against PCSCs has already been demonstrated (for example resveratrol, quercetin, and green tea catechins, and curcumin)[92], and for this reason they would deserve to be analysed as nanoparticle formulations. Among these natural compounds there are withaferin A (a major component of Withania somnifora) and carnosol (found in Rosmarinus officinalis, Salvia carnosa, and Origanum vulgare). They have suppressive effects on the proliferation, migration, and activation of c-Met in PCSCs[93]. A recent study investigated the role of α-mangostin (derived from the plant mangosteen) encapsulated NPs (Mang-NPs) in the inhibition of pancreatic carcinogenesis by targeting CSCs in human and transgenic mice. The data obtained indicated that Mang-NPs suppress PCSCs features (i.e., EMT, cell proliferation, cell cycle, pluripotency, self-renewal, and apoptosis) and also target CSCs in mice[94]. A similar approach has been implemented for the investigation of the efficacy of anthothecol (an antimalarial compound) encapsulated by PLGA NPs (antho-NPs) against PCSCs. Interestingly, it has been demonstrated that antho-NPs specifically inhibit PCSCs growth by modulating the SHH pathway[95].
Although significant progress has been made in the development of NPs, they are far from optimal. Indeed, there are already problems regarding the low drug loading capacity of some NPs. Liposomes are sometimes affected by drug diffusion through the liposome bilayer, and micellar drugs exhibit in vivo instability[90]. For these reasons, polymeric and nanogel drug conjugates, characterized by controlled drug release and higher drug loading capacity, provide a better strategy. Other challenges that must be addressed in the future for clinical use of NPs concern inefficient delivery, inherent toxicity, off-target effects, unfavourable biological distribution, and lack of clearance from the systemic circulation[96]. In conclusion, even if further research is needed for the development of efficient NPs, it is possible to speculate that the targeted delivery system for anti-cancer agents will be translated into clinical practice. It is tempting to imagine that in the near future modified NPs might serve as promising nanocarriers for site-specific drug delivery by targeting PCSCs and that protocol might be further improved for in vivo applications.
In conclusion, although further studies are needed, the new developments in targeting PCSCs are expected to have high impact in the treatment of PDAC in coming years. Nevertheless, some questions still need further investigation. While PCSCs represent an intriguing target for therapy, their complete characterisation is still needed. The identification of proteomic profiles and in particular of the deregulated pathways and proteins of PCSCs is fundamental to increasing our knowledge about pancreatic cancer and to identify new therapeutic approaches to eradicate PDAC stem cells that result in recurrence of the disease. Thus, enhanced biological knowledge of PCSCs, combined with the development of nanoparticle technology, promises to be key for the development of new effective treatments of pancreatic cancer.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hassan AI, Oltra E, Pelagalli A, Saeki K S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Gallmeier E, Gress TM. [Pancreatic ductal adenocarcinoma]. Internist (Berl). 2018;59:805-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Luchini C, Capelli P, Scarpa A. Pancreatic Ductal Adenocarcinoma and Its Variants. Surg Pathol Clin. 2016;9:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018;8:pii: a031435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 4. | Houg DS, Bijlsma MF. The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma. Mol Cancer. 2018;17:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1346] [Article Influence: 149.6] [Reference Citation Analysis (2)] |
| 6. | Guseva LN. [Nitritometric determination of allacyl]. Farmatsiia. 1969;18:43-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Ellenrieder V, König A, Seufferlein T. Current Standard and Future Perspectives in First- and Second-Line Treatment of Metastatic Pancreatic Adenocarcinoma. Digestion. 2016;94:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q, Yu X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp Mol Med. 2017;49:e406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel). 2017;9:pii: E157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 11. | Al Haddad AH, Adrian TE. Challenges and future directions in therapeutics for pancreatic ductal adenocarcinoma. Expert Opin Investig Drugs. 2014;23:1499-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Valle S, Martin-Hijano L, Alcalá S, Alonso-Nocelo M, Sainz B Jr. The Ever-Evolving Concept of the Cancer Stem Cell in Pancreatic Cancer. Cancers (Basel). 2018;10:pii: E33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Santamaria S, Delgado M, Kremer L, Garcia-Sanz JA. Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible? Front Immunol. 2017;8:1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1183] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 15. | Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park). 2014;28:1101-1107, 1110. [PubMed] |
| 16. | Dubarry JJ, Quinton A, Bancons J. [Use of colopten in intestinal pathology]. Bord Med. 1971;4:561-564 passim. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Liu P, Wang Z, Brown S, Kannappan V, Tawari PE, Jiang W, Irache JM, Tang JZ, Armesilla AL, Darling JL. Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget. 2014;5:7471-7485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Lei Y, Zhang D, Yu J, Dong H, Zhang J, Yang S. Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett. 2017;393:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol. 2015;31:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2428] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 21. | Dalla Pozza E, Dando I, Biondani G, Brandi J, Costanzo C, Zoratti E, Fassan M, Boschi F, Melisi D, Cecconi D. Pancreatic ductal adenocarcinoma cell lines display a plastic ability to bidirectionally convert into cancer stem cells. Int J Oncol. 2015;46:1099-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Ishiwata T, Matsuda Y, Yoshimura H, Sasaki N, Ishiwata S, Ishikawa N, Takubo K, Arai T, Aida J. Pancreatic cancer stem cells: features and detection methods. Pathol Oncol Res. 2018;24:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Rao CV, Mohammed A. New insights into pancreatic cancer stem cells. World J Stem Cells. 2015;7:547-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Chalquest RR. Preveterinary requirements and admission to American veterinary colleges: important changes. J Am Vet Med Assoc. 1986;189:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Biondani G, Zeeberg K, Greco MR, Cannone S, Dando I, Dalla Pozza E, Mastrodonato M, Forciniti S, Casavola V, Palmieri M. Extracellular matrix composition modulates PDAC parenchymal and stem cell plasticity and behavior through the secretome. FEBS J. 2018;285:2104-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Subramaniam D, Kaushik G, Dandawate P, Anant S. Targeting Cancer Stem Cells for Chemoprevention of Pancreatic Cancer. Curr Med Chem. 2018;25:2585-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Brandi J, Dalla Pozza E, Dando I, Biondani G, Robotti E, Jenkins R, Elliott V, Park K, Marengo E, Costello E. Secretome protein signature of human pancreatic cancer stem-like cells. J Proteomics. 2016;136:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Ercan G, Karlitepe A, Ozpolat B. Pancreatic Cancer Stem Cells and Therapeutic Approaches. Anticancer Res. 2017;37:2761-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Renz BW, D’Haese JG, Werner J, Westphalen CB, Ilmer M. Repurposing Established Compounds to Target Pancreatic Cancer Stem Cells (CSCs). Med Sci (Basel). 2017;5:pii: E14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Meidhof S, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M, Schüler J, Berthold M, Weber A, Burk U. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med. 2015;7:831-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1357] [Cited by in RCA: 1638] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 32. | Zhan HX, Xu JW, Wu D, Zhang TP, Hu SY. Pancreatic cancer stem cells: new insight into a stubborn disease. Cancer Lett. 2015;357:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Najumudeen AK, Jaiswal A, Lectez B, Oetken-Lindholm C, Guzmán C, Siljamäki E, Posada IM, Lacey E, Aittokallio T, Abankwa D. Cancer stem cell drugs target K-ras signaling in a stemness context. Oncogene. 2016;35:5248-5262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Schenk M, Aykut B, Teske C, Giese NA, Weitz J, Welsch T. Salinomycin inhibits growth of pancreatic cancer and cancer cell migration by disruption of actin stress fiber integrity. Cancer Lett. 2015;358:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6:4569-4584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 377] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 36. | Skrtić M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 534] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 37. | Reed GA, Schiller GJ, Kambhampati S, Tallman MS, Douer D, Minden MD, Yee KW, Gupta V, Brandwein J, Jitkova Y. A Phase 1 study of intravenous infusions of tigecycline in patients with acute myeloid leukemia. Cancer Med. 2016;5:3031-3040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Zhou HM, Dong TT, Wang LL, Feng B, Zhao HC, Fan XK, Zheng MH. Suppression of colorectal cancer metastasis by nigericin through inhibition of epithelial-mesenchymal transition. World J Gastroenterol. 2012;18:2640-2648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Balic A, Sørensen MD, Trabulo SM, Sainz B Jr, Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J, Erkan M, Heeschen C. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther. 2014;13:1758-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Fiorillo M, Lamb R, Tanowitz HB, Mutti L, Krstic-Demonacos M, Cappello AR, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Repurposing atovaquone: targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget. 2016;7:34084-34099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 41. | Garnier A, Vykoukal J, Hubertus J, Alt E, von Schweinitz D, Kappler R, Berger M, Ilmer M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int J Oncol. 2015;47:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Blaj C, Bringmann A, Schmidt EM, Urbischek M, Lamprecht S, Fröhlich T, Arnold GJ, Krebs S, Blum H, Hermeking H. ADNP Is a Therapeutically Inducible Repressor of WNT Signaling in Colorectal Cancer. Clin Cancer Res. 2017;23:2769-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Malsy M, Gebhardt K, Gruber M, Wiese C, Graf B, Bundscherer A. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol. 2015;15:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Zhang Y, Liu L, Fan P, Bauer N, Gladkich J, Ryschich E, Bazhin AV, Giese NA, Strobel O, Hackert T. Aspirin counteracts cancer stem cell features, desmoplasia and gemcitabine resistance in pancreatic cancer. Oncotarget. 2015;6:9999-10015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Coyle C, Cafferty FH, Rowley S, MacKenzie M, Berkman L, Gupta S, Pramesh CS, Gilbert D, Kynaston H, Cameron D. ADD-ASPIRIN: A phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp Clin Trials. 2016;51:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 46. | Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol. 2013;6:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 48. | Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 49. | Owunari GU, Minakiri SI. Disulfiram and copper gluconate in cancer chemotherapy; a review of the literature. Cancer Res. 2014;2:88-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Kim SK, Kim H, Lee DH, Kim TS, Kim T, Chung C, Koh GY, Kim H, Lim DS. Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One. 2013;8:e78130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Han D, Wu G, Chang C, Zhu F, Xiao Y, Li Q, Zhang T, Zhang L. Disulfiram inhibits TGF-β-induced epithelial-mesenchymal transition and stem-like features in breast cancer via ERK/NF-κB/Snail pathway. Oncotarget. 2015;6:40907-40919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Cong J, Wang Y, Zhang X, Zhang N, Liu L, Soukup K, Michelakos T, Hong T, DeLeo A, Cai L. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett. 2017;409:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, Nisman B, Peretz T, Peylan-Ramu N. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. 2015;20:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Higashi T, Hayashi H, Kitano Y, Yamamura K, Kaida T, Arima K, Taki K, Nakagawa S, Okabe H, Nitta H. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Rangarajan P, Subramaniam D, Paul S, Kwatra D, Palaniyandi K, Islam S, Harihar S, Ramalingam S, Gutheil W, Putty S. Crocetinic acid inhibits hedgehog signaling to inhibit pancreatic cancer stem cells. Oncotarget. 2015;6:27661-27673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Ma Y, Yu W, Shrivastava A, Alemi F, Lankachandra K, Srivastava RK, Shankar S. Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis. 2017;38:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 57. | Miyazaki Y, Matsubara S, Ding Q, Tsukasa K, Yoshimitsu M, Kosai K, Takao S. Efficient elimination of pancreatic cancer stem cells by hedgehog/GLI inhibitor GANT61 in combination with mTOR inhibition. Mol Cancer. 2016;15:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One. 2014;9:e91983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Ponnurangam S, Dandawate PR, Dhar A, Tawfik OW, Parab RR, Mishra PD, Ranadive P, Sharma R, Mahajan G, Umar S. Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells. Oncotarget. 2016;7:3217-3232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Matsubara S, Ding Q, Miyazaki Y, Kuwahata T, Tsukasa K, Takao S. mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci Rep. 2013;3:3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Zeng JY, Sharma S, Zhou YQ, Yao HP, Hu X, Zhang R, Wang MH. Synergistic activities of MET/RON inhibitor BMS-777607 and mTOR inhibitor AZD8055 to polyploid cells derived from pancreatic cancer and cancer stem cells. Mol Cancer Ther. 2014;13:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS One. 2016;11:e0147717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 63. | Bizzarro V, Belvedere R, Milone MR, Pucci B, Lombardi R, Bruzzese F, Popolo A, Parente L, Budillon A, Petrella A. Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget. 2015;6:25076-25092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Fang Y, Guan X, Cai T, Long J, Wang H, Xie X, Zhang Y. Knockdown of ANXA1 suppresses the biological behavior of human NSCLC cells in vitro. Mol Med Rep. 2016;13:3858-3866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Fong LWR, Yang DC, Chen CH. Myristoylated alanine-rich C kinase substrate (MARCKS): a multirole signaling protein in cancers. Cancer Metastasis Rev. 2017;36:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Chen CH, Thai P, Yoneda K, Adler KB, Yang PC, Wu R. A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis. Oncogene. 2014;33:3696-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Song S, Ji B, Ramachandran V, Wang H, Hafley M, Logsdon C, Bresalier RS. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS One. 2012;7:e42699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 68. | Kang HG, Kim DH, Kim SJ, Cho Y, Jung J, Jang W, Chun KH. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget. 2016;7:68229-68241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Nangia-Makker P, Hogan V, Raz A. Galectin-3 and cancer stemness. Glycobiology. 2018;28:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 70. | Zhang L, Wang P, Qin Y, Cong Q, Shao C, Du Z, Ni X, Li P, Ding K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene. 2017;36:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Glinsky VV, Kiriakova G, Glinskii OV, Mossine VV, Mawhinney TP, Turk JR, Glinskii AB, Huxley VH, Price JE, Glinsky GV. Synthetic galectin-3 inhibitor increases metastatic cancer cell sensitivity to taxol-induced apoptosis in vitro and in vivo. Neoplasia. 2009;11:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Shankar Babu M, Mahanta S, Lakhter AJ, Hato T, Paul S, Naidu SR. Lapachol inhibits glycolysis in cancer cells by targeting pyruvate kinase M2. PLoS One. 2018;13:e0191419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Xie X, Huang X, Tang H, Ye F, Yang L, Guo X, Tian Z, Xie X, Peng C, Xie X. Diallyl Disulfide Inhibits Breast Cancer Stem Cell Progression and Glucose Metabolism by Targeting CD44/PKM2/AMPK Signaling. Curr Cancer Drug Targets. 2018;18:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Ning X, Qi H, Li R, Jin Y, McNutt MA, Yin Y. Synthesis and antitumor activity of novel 2, 3-didithiocarbamate substituted naphthoquinones as inhibitors of pyruvate kinase M2 isoform. J Enzyme Inhib Med Chem. 2018;33:126-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Talaiezadeh A, Shahriari A, Tabandeh MR, Fathizadeh P, Mansouri S. Kinetic characterization of lactate dehydrogenase in normal and malignant human breast tissues. Cancer Cell Int. 2015;15:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Fiume L, Manerba M, Vettraino M, Di Stefano G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med Chem. 2014;6:429-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Altamimi AS, Alafeefy AM, Balode A, Vozny I, Pustenko A, El Shikh ME, Alasmary FAS, Abdel-Gawad SA, Žalubovskis R. Symmetric molecules with 1,4-triazole moieties as potent inhibitors of tumour-associated lactate dehydrogenase-A. J Enzyme Inhib Med Chem. 2018;33:147-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 79. | Tabatabaei-Dakhili SA, Aguayo-Ortiz R, Domínguez L, Velázquez-Martínez CA. Untying the knot of transcription factor druggability: Molecular modeling study of FOXM1 inhibitors. J Mol Graph Model. 2018;80:197-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Kida YS, Kawamura T, Wei Z, Sogo T, Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR. ERRs Mediate a Metabolic Switch Required for Somatic Cell Reprogramming to Pluripotency. Cell Stem Cell. 2015;16:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 81. | Yu DD, Huss JM, Li H, Forman BM. Identification of novel inverse agonists of estrogen-related receptors ERRγ and ERRβ. Bioorg Med Chem. 2017;25:1585-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Kim JH, Choi YK, Byun JK, Kim MK, Kang YN, Kim SH, Lee S, Jang BK, Park KG. Estrogen-related receptor γ is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp Mol Med. 2016;48:e213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Singh TD, Jeong SY, Lee SW, Ha JH, Lee IK, Kim SH, Kim J, Cho SJ, Ahn BC, Lee J. Inverse Agonist of Estrogen-Related Receptor γ Enhances Sodium Iodide Symporter Function Through Mitogen-Activated Protein Kinase Signaling in Anaplastic Thyroid Cancer Cells. J Nucl Med. 2015;56:1690-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 85. | Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, Jadidi-Niaragh F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett. 2017;190:64-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 86. | Golchin A, Hosseinzadeh S, Roshangar L. The role of nanomaterials in cell delivery systems. Med Mol Morphol. 2018;51:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Aftab S, Shah A, Nadhman A, Kurbanoglu S, Aysıl Ozkan S, Dionysiou DD, Shukla SS, Aminabhavi TM. Nanomedicine: An effective tool in cancer therapy. Int J Pharm. 2018;540:132-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 88. | Yang F, Jin C, Subedi S, Lee CL, Wang Q, Jiang Y, Li J, Di Y, Fu D. Emerging inorganic nanomaterials for pancreatic cancer diagnosis and treatment. Cancer Treat Rev. 2012;38:566-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Yang F, Jin C, Jiang Y, Li J, Di Y, Ni Q, Fu D. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer Treat Rev. 2011;37:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 90. | Wei X, Senanayake TH, Warren G, Vinogradov SV. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug Chem. 2013;24:658-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 91. | Saneja A, Nayak D, Srinivas M, Kumar A, Khare V, Katoch A, Goswami A, Vishwakarma RA, Sawant SD, Gupta PN. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur J Pharm Sci. 2017;97:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Gupta S, Pramanik D. Phytochemicals and cancer stem cells: A pancreatic cancer overview. Current Chemical Biology. 2016;10:10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Aliebrahimi S, Kouhsari SM, Arab SS, Shadboorestan A, Ostad SN. Phytochemicals, withaferin A and carnosol, overcome pancreatic cancer stem cells as c-Met inhibitors. Biomed Pharmacother. 2018;106:1527-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 94. | Verma RK, Yu W, Shrivastava A, Shankar S, Srivastava RK. α-Mangostin-encapsulated PLGA nanoparticles inhibit pancreatic carcinogenesis by targeting cancer stem cells in human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice. Sci Rep. 2016;6:32743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Verma RK, Yu W, Singh SP, Shankar S, Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomedicine. 2015;11:2061-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Li J, Liu F, Gupta S, Li C. Interventional Nanotheranostics of Pancreatic Ductal Adenocarcinoma. Theranostics. 2016;6:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Wadler S, Tenteromano L, Cazenave L, Sparano JA, Greenwald ES, Rozenblit A, Kaleya R, Wiernik PH. Phase II trial of echinomycin in patients with advanced or recurrent colorectal cancer. Cancer Chemother Pharmacol. 1994;34:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 98. | Muss HB, Blessing JA, DuBeshter B. Echinomycin in recurrent and metastatic endometrial carcinoma. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1993;16:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |