Published online Dec 31, 2009. doi: 10.4252/wjsc.v1.i1.36
Revised: October 15, 2009
Accepted: October 22, 2009
Published online: December 31, 2009
The success achieved over the last decade with islet transplantation has intensified interest in treating diabetes, not only by cell transplantation, but also by stem cells. The formation of insulin-producing cells from pancreatic duct, acinar, and liver cells is an active area of investigation. Protocols for the in vitro differentiation of embryonic stem (ES) cells based on normal developmental processes, have generated insulin-producing cells, though at low efficiency and without full responsiveness to extracellular levels of glucose. Induced pluripotent stem cells, which have been generated from somatic cells by introducing Oct3/4, Sox2, Klf4, and c-Myc, and which are similar to ES cells in morphology, gene expression, epigenetic status and differentiation, can also differentiate into insulin-producing cells. Overexpression of embryonic transcription factors in stem cells could efficiently induce their differentiation into insulin-expressing cells. The purpose of this review is to demonstrate recent progress in the research for new sources of β-cells, and to discuss strategies for the treatment of diabetes.
- Citation: Noguchi H. Recent advances in stem cell research for the treatment of diabetes. World J Stem Cells 2009; 1(1): 36-42
- URL: https://www.wjgnet.com/1948-0210/full/v1/i1/36.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v1.i1.36
The pancreas is a mixed exocrine and endocrine grand that controls many homeostatic functions. The exocrine pancreas consists of acinar cells and the ductal epithelium, while the endocrine pancreas consists of four cell types (α-, β-, δ-, and pancreatic-polypeptide cells). The pancreas controls body metabolism, including the digestion of foods by exocrine enzymes secreted from acinar cells and the regulation of blood glucose levels by insulin secreted from β-cells. Clinical studies have shown that transplantation of a pancreas or purified pancreatic islets can support glucose homeostasis in type 1 diabetic individuals, in whom the β-cells have been destroyed by an autoimmune reaction[1-5]. Islet transplantation carries the special advantages of being less invasive and resulting in fewer complications compared with the traditional pancreas or pancreas-kidney transplantation. However, islet transplantation efforts have limitations including the short supply of donor pancreata, the paucity of experienced islet isolation teams, side effects of immunosuppressants and poor long-term results[6]. These limitations have led to the search for other stem/progenitor cell sources of β-cells and intense interest in how the differentiation of such progenitors can be directed, or “programmed”, efficiently. The programming efforts are based on understanding how β-cells are normally generated in the embryo and how they arise during regeneration in adults, in response to tissue damage and disease. Here, we review recent studies on β-cell development and regeneration, and highlight unresolved issues in the field.
The pancreas is specified from the endoderm germ layer and develops from a dorsal and ventral protrusion of the primitive gut epithelium[7-9]. These two pancreatic buds grow, branch, and fuse to form the definitive pancreas. Initially, broad suppression of mesodermal Wnt and fibroblast growth factor (FGF) signaling in the foregut enables pancreas induction, whereas active mesodermal Wnt signaling in the posterior gut suppresses these tissue fates[10,11]. Retinoic acid signaling, apparently from paraxial mesoderm cells, helps to further refine the anterior-posterior position, in which the liver and pancreas can develop from the gut endoderm[12-15]. Subsequently, in the ventral foregut, FGF from the cardiac mesoderm and bone morphogenetic protein/transforming growth factor-β (BMP/TGF-β) from septum transversum mesenchyme cells coordinately induce the liver program and suppress the pancreas program[16-19]. In the dorsal foregut, signals from the notochord, including activin and FGF, suppress sonic hedgehog (shh) signaling within the endoderm and allow the pancreatic program[20,21].
The newly specified pancreatic endoderm is initially marked by the expression of the pancreatic and duodenal homeobox gene 1 (Pdx1; also known as Ipf1) and then by the pancreas specific transcription factor 1a (Ptf1a)[22,23]. Both proteins are crucial for pancreatic development. Pdx1 marks all pancreatic and midgut progenitors[22] and is crucial for development after the bud stage[24,25]. Pdx1 levels also help to control the balance between the endocrine and exocrine (acinar and duct) progenitors that differentiate within the pancreas[26]. Notch signaling also helps to regulate the balance of exocrine and endocrine cells, probably by allowing the expansion of an undifferentiated pancreatic-progenitor population[27-29]. Loss of Notch signaling allows the endocrine lineage to develop, which is marked by and requires the basic helix-loop-helix (bHLH) transcription factor, neurogenin 3 (Ngn3)[22,27,30]. Ngn3 directly influences the expression of another islet specific bHLH gene, neurogenic differentiation (NeuroD; also known as BETA2)[31]. A loss of function assay of NeuroD/BETA2 implicates a phenotype similar to, but less severe than, Ngn3, leading to a diminished number of all endocrine cell types[32]. Then, definitive β cells are generated under the influence of the v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) transcription factor[33,34].
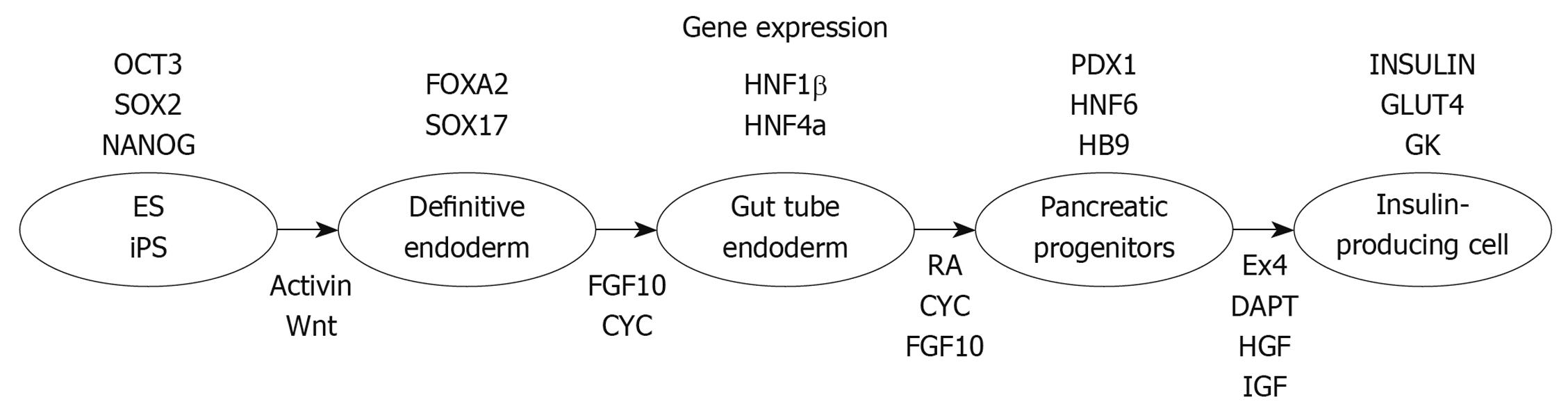
ES cells, which are pluripotent diploid cells and can be induced to differentiate into cells of all three germ layers both in vivo and in vitro[35,36], are a potentially abundant source of β-cells. It has been reported that ES cells from mouse[37-40], monkey[41], and human[38,42] were able to differentiate into insulin-positive cells, a potential source of new β-cells. Numerous groups have been developing ES cell differentiation protocols that attempt to mimic normal embryonic development. The first step of pancreatic development is the induction of a definitive endoderm using high concentrations of activin A treatment[43,44]. Further treatment in sequential stages with keratinocyte growth factor (KGF), retinoic acid, Noggin, and cyclopamine (the hedgehog-signaling inhibitor) can then direct definitive endoderm toward Pdx1-expressing posterior foregut endoderm cells[45,46]. Treatment with DAPT and exendin-4 recruited the Pdx1-expressing posterior foregut endoderm cells to the pancreatic and endocrine lineages, which expressed Pdx1, Nkx6-1, Nkx2-2, Ngn3, and/or Pax4. After treatment with exendin-4, IGF-1, and HGF, endocrine cells expressing the pancreatic hormones insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin are produced (Figure 1).
Melton’s group recently reported small molecules that efficiently direct endodermal differentiation of mouse and human embryonic stem cells[47]. In a screen of 4000 compounds, they identified two cell-permeable small molecules that direct differentiation of ES cells into the endodermal lineage. The efficiency of differentiation into definitive endoderm using these compounds was higher than that achieved by Activin A or Nodal, which commonly used protein inducers of endoderm. The definitive endoderm induced by these compounds was able to participate in normal development when injected into developing embryos, and was able to form pancreatic progenitors. These small molecules could induce reproducible and efficient differentiation of ES cells into endoderm.
On the other hand, a significant number of problems remain unsolved in terms of clinical application of ES cells, such as the risk of tumorigenicity and immunosuppression after transplantation. The ethical issue is another major obstacle to the clinical use of ES cells.
iPS cells are also pluripotent diploid cells that can be induced to differentiate into cells of all three germ layers both in vivo and in vitro. Moreover, iPS cells have fewer ethical issues compared with ES cells, because iPS cells can be established from somatic cells. Initial iPS cells have been generated from mouse and human somatic cells by introducing Oct3/4 and Sox2 with either Klf4 and c-Myc or Nanog and Lin28, using retroviruses[48-51]. Recently, it has been reported that valproic acid (VPA), a histone deacetylase inhibitor, enables reprogramming of primary human fibroblasts with only two factors, Oct4 and Sox2, without the need for the c-Myc or Klf4[52]. The results support the possibility of reprogramming by chemical means, which would make therapeutic use of reprogrammed cells safer and more practical. Another group showed that adult mouse neural stem cells (NSCs) expressed higher endogenous levels of Sox2 and c-Myc than embryonic stem cells[53] and that exogenous expression of the germline-specific transcription factor Oct4 was sufficient to generate pluripotent stem cells from adult mouse NSCs[54]. These data suggest that, in inducing pluripotency, the number of reprogramming factors can be reduced when using somatic cells that endogenously express appropriate levels of complementing factors.
On the other hand, retroviral integration of the transcription factors might activate or inactivate host genes, resulting in tumorigenecity, as was the case in some patients who underwent gene therapy. Yamanaka’s group reported the generation of mouse iPS cells with transient expressions of Oct3/4, Sox2, Klf4, and c-Myc from plasmids. Repeated transfection into mouse embryonic fibroblasts of two expression plasmids, one containing complementary DNAs (cDNAs) for Oct3/4, Sox2, and Klf4 and the other containing the c-Myc cDNA, resulted in iPS cells without evidence of plasmid integration[55]. At the same time, another group demonstrated the generation of mouse iPS cells from fibroblasts and liver cells using non-integrating adenoviruses transiently expressing Oct4, Sox2, Klf4, and c-Myc[56]. Moreover, Ding’s groupreported generation of protein-induced pluripotent stem cells (piPSCs) from murine embryonic fibroblasts using recombinant cell-penetrating reprogramming proteins without transfection of any genes[57]. For efficient transduction of four reprogramming factors, Oct4, Sox2, Klf4, and c-Myc, into cells, they used protein transduction technology[58-61]. A poly-arginine (11R) protein transduction domain (PTD) fused to the C terminus of these reprogramming factors efficiently delivered the proteins into cells and induced iPS cells, which demonstrated long-term self-renewal and were pluripotent in vitro and in vivo. These reports provide strong evidence that insertional mutagenesis is not required for in vitro reprogramming. The production of iPS cells without integration into the host genome addresses a critical safety concern for potential use of iPS cells in regenerative medicine.
Although some papers have shown the generation of insulin-secreting islet-like clusters from human iPS cells[62,63], the efficiency of the method seems low. The method, as detailed in this review in the ES cells section, might represent a critical step in the development of insulin-producing cells from iPS cells (Figure 1). Indeed, Melton’s group recently reported generation of iPS cells from patients with type 1 diabetes and differentiation from the iPS cells into insulin-producing cells using this method[64].
Although it is clear that the majority of new β-cells derive from pre-existing insulin-expressing cells after surgical injury[65,66], several in vitro studies have shown that insulin-producing cells can be generated from adult pancreatic ductal tissues[67-71]. A recent study has shown that duct ligation can activate Ngn3-positive β-cell precursors in the ductal epithelium[72]. The Edmonton group has shown that, in clinical islet transplantation, a significant positive correlation exists between the number of ductal-epithelial cells transplanted and long-term metabolic success, as assessed by an intravenous glucose tolerance test at approximately two years post-transplantation. No significant correlation was observed between the total islet equivalents and long-term metabolic success[73]. Cells in the pancreatic anlage migrate from the ducts while differentiating to form clusters that will eventually become islets during embryonic development [74]; therefore, the post-natal pancreatic duct might harbor islet precursor/stem cells. Inada et al[75] generated transgenic mice expressing human carbonic anhydrase II (CAII) promoter-Cre recombinase or inducible CreER to cross with ROSA26 loxP-Stop-loxP LacZ reporter mice. CAII-expressing cells within the pancreas act as progenitors that give rise to both new islets and acini normally after birth and after injury (ductal ligation). This identification of a differentiated pancreatic cell type as an in vivo progenitor of all differentiated pancreatic cell types has implications for a potential expandable source of new islets for replenishment therapy for diabetes[75]. Such interesting results suggest the possibility of multipotent progenitors in adult pancreatic ducts.
Mouse pancreatic stem cells have been isolated from duct-rich population, which are capable of self-renewal and multipotency[76,77]. On the other hand, human cells from the duct-rich population were unable to divide after 30 d under several culture conditions, although the cells were able to differentiate into insulin-producing cells[78]. There are some differences between the methodologies used by the two groups, such as culture conditions, isolation stresses, and/or species themselves. The ability of β-cells to expand is limited, especially in the adult, and the partial growth ability is insufficient to permit recovery from cell loss in type 1 diabetes[79]. Therefore, it is important to isolate human pancreatic “stem” cells comprising a sufficient number of β-cells for the treatment of diabetes.
The transdifferentiation of acinar cells to islets has also been proposed[80-82]. Melton’s group showed in vivo reprogramming of adult pancreatic exocrine cells to β-cells by viral delivery of the developmental transcription factors Pdx1, Ngn3, and MafA[83]. Pancreatic exocrine cells greatly outnumber β-cells; therefore, the transdifferentiation of acinar cells to β-cells is also an interesting possibility.
Another interesting stem cell in this field is the mesenchymal stem cell (MSC). It has been reported that marginal mass islet transplantation with autologous MSCs promotes long-term islet allograft survival and sustained normoglycemia[84]. MSCs also prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9[85], and protect NOD mice from diabetes by inducing regulatory T cells[86].
Several reports have suggested that epitopic transdifferentiation is also possible. In vivo transduction of mice with an adenovirus expressing Pdx-1[87,88], and both betacellulin and NeuroD[89], or a modified form of Pdx-1 carrying the VP16 transcriptional activation domain[90], or MafA together with Pdx-1 and NeuroD[91], markedly increased insulin biosynthesis and induced various pancreas-related factors in the liver. The existence of potential β-cell precursors in the adult liver is of obvious medical interest. Moreover, overexpression of embryonic transcription factors in stem cells could efficiently induce their differentiation into insulin-expressing cells. We reported that transduction of Pdx-1 and NeuroD proteins induces insulin gene expression[67,92,93]. Other groups also showed that transduction of NeuroD in vivo or TAT-Ngn3 fused TAT-PTD induced insulin-producing cells[94,95]. The production of insulin-producing cells using protein transduction technology without gene transduction addresses a critical safety concern for potential use of the cells in regenerative medicine. Further investigations to induce differentiation of stem/progenitor cells into insulin-producing cells will help to establish cell-based therapies in diabetes.
Peer reviewer: Richard Schäfer, MD, Specialist for Internal Medicine and Transfusion Medicine, Head Mesenchymal Stem Cell Laboratory, Institute of Clinical and Experimental Transfusion Medicine, Eberhard Karls University Tübingen, Otfried-Müller-Str. 4/1, D-72076 Tübingen, Germany
S- Editor Li LF L- Editor Stewart GJ E- Editor Lin YP
| 1. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. |
| 2. | Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Nagata H, Yonekawa Y, Yamada Y, Fukuda K, Tsukiyama K, Suzuki H. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet. 2005;365:1642-1644. |
| 3. | Noguchi H, Iwanaga Y, Okitsu T, Nagata H, Yonekawa Y, Matsumoto S. Evaluation of islet transplantation from non-heart beating donors. Am J Transplant. 2006;6:2476-2482. |
| 4. | Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5:2037-2046. |
| 5. | Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830-835. |
| 6. | Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694-705. |
| 7. | Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524-532. |
| 8. | Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490-1494. |
| 9. | Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet. 2008;9:329-340. |
| 10. | McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207-2217. |
| 11. | Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563-1572. |
| 12. | Chen Y, Pan FC, Brandes N, Afelik S, Sölter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144-160. |
| 13. | Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109-122. |
| 14. | Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dollé P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399-411. |
| 15. | Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949-956. |
| 16. | Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871-881. |
| 17. | Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998-2003. |
| 18. | Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998-2009. |
| 19. | Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DY. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041-2050. |
| 20. | Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801-804. |
| 21. | Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705-1713. |
| 22. | Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447-2457. |
| 23. | Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128-134. |
| 24. | Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606-609. |
| 25. | Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983-995. |
| 26. | Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253-266. |
| 27. | Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877-881. |
| 28. | Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36-44. |
| 29. | Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algül H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757-2765. |
| 30. | Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607-1611. |
| 31. | Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000;20:3292-3307. |
| 32. | Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323-2334. |
| 33. | Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049-6062. |
| 34. | Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA. 2002;99:6737-6742. |
| 35. | Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699-708. |
| 36. | Soto-Gutiérrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412-1419. |
| 37. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. |
| 38. | Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697. |
| 39. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. |
| 40. | Moritoh Y, Yamato E, Yasui Y, Miyazaki S, Miyazaki J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003;52:1163-1168. |
| 41. | Lester LB, Kuo HC, Andrews L, Nauert B, Wolf DP. Directed differentiation of rhesus monkey ES cells into pancreatic cell phenotypes. Reprod Biol Endocrinol. 2004;2:42. |
| 42. | Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265-274. |
| 43. | D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534-1541. |
| 44. | Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713-R724. |
| 45. | D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. |
| 46. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. |
| 47. | Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348-358. |
| 48. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. |
| 49. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. |
| 50. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. |
| 51. | Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317. |
| 52. | Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269-1275. |
| 53. | Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646-650. |
| 54. | Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411-419. |
| 55. | Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949-953. |
| 56. | Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945-949. |
| 57. | Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381-384. |
| 58. | Noguchi H, Matsumoto S. Protein transduction technology offers a novel therapeutic approach for diabetes. J Hepatobiliary Pancreat Surg. 2006;13:306-313. |
| 59. | Noguchi H, Matsumoto S. Protein transduction technology: a novel therapeutic perspective. Acta Med Okayama. 2006;60:1-11. |
| 60. | Noguchi H, Matsumoto S, Onaca N, Naziruddin B, Jackson A, Ikemoto T, Shimoda M, Fujita Y, Chujo D, Iwanaga Y. Ductal injection of JNK inhibitors before pancreas preservation prevents islet apoptosis and improves islet graft function. Hum Gene Ther. 2009;20:73-85. |
| 61. | Noguchi H, Nakai Y, Ueda M, Masui Y, Futaki S, Kobayashi N, Hayashi S, Matsumoto S. Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia. 2007;50:612-619. |
| 62. | Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601-31607. |
| 63. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. |
| 64. | Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768-15773. |
| 65. | Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41-46. |
| 66. | Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817-826. |
| 67. | Noguchi H, Kaneto H, Weir GC, Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes. 2003;52:1732-1737. |
| 68. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. |
| 69. | Heremans Y, Van De Casteele M, in't Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303-312. |
| 70. | Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes. 2004;53:2143-2152. |
| 71. | Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720-732. |
| 72. | Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197-207. |
| 73. | Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107-3114. |
| 74. | Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35-43. |
| 75. | Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915-19919. |
| 76. | Yamamoto T, Yamato E, Taniguchi H, Shimoda M, Tashiro F, Hosoi M, Sato T, Fujii S, Miyazaki JI. Stimulation of cAMP signalling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia. 2006;49:2359-2367. |
| 77. | Noguchi H, Oishi K, Ueda M, Yukawa H, Hayashi S, Kobayashi N, Levy MF, Matusmoto S. Establishment of mouse pancreatic stem cell line. Cell Transplant. 2009;18:563-571. |
| 78. | Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N. Characterization of human pancreatic progenitor cells. Cell Transplant. 2010;In press. |
| 79. | Nishio J, Gaglia JL, Turvey SE, Campbell C, Benoist C, Mathis D. Islet recovery and reversal of murine type 1 diabetes in the absence of any infused spleen cell contribution. Science. 2006;311:1775-1778. |
| 80. | Lardon J, Huyens N, Rooman I, Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch. 2004;444:61-65. |
| 81. | Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49-57. |
| 82. | Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA. 2005;102:15116-15121. |
| 83. | Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627-632. |
| 84. | Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32:116-124. |
| 85. | Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797-1806. |
| 86. | Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A, Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391-1399. |
| 87. | Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568-572. |
| 88. | Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950-31957. |
| 89. | Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596-603. |
| 90. | Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009-1022. |
| 91. | Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, Yamasaki Y. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005;280:15047-15052. |
| 92. | Noguchi H, Bonner-Weir S, Wei FY, Matsushita M, Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes. 2005;54:2859-2866. |
| 93. | Noguchi H, Ueda M, Matsumoto S, Kobayashi N, Hayashi S. BETA2/NeuroD protein transduction requires cell surface heparan sulfate proteoglycans. Hum Gene Ther. 2007;18:10-17. |
| 94. | Huang Y, Chen J, Li G, Cheng TY, Jiang MH, Zhang SY, Lu J, Yan S, Fan WW, Lu DR. Reversal of hyperglycemia by protein transduction of NeuroD in vivo. Acta Pharmacol Sin. 2007;28:1181-1188. |