INTRODUCTION
Genetically altered mice offer researchers a powerful means to dissect and understand complex biological processes, as well as to manipulate gene expression, with the ultimate goal of developing therapeutic strategies for a variety of diseases including cancer, inflammatory and infectious diseases, and neurogenetic and cardiovascular disorders[1-5].
These mice are typically generated by means of the introduction of a specific population of cells called “embryonic stem cells”, into a host preimplantational embryo. Embryonic Stem cells (ES cells) can self-replicate and are pluripotent. Originally, ES cells were isolated from blastocyst stage embryos[6], but recently a new method for generation of induced pluripotent stem (iPS) cells from somatic cells has been developed[7]. iPS cells are derived from autologous somatic cells after genetic reprogramming and were first described by Takahashi et al[7] and were independently confirmed later by others. Recently, iPS technology has opened new avenues for the generation of animals from genetically-modified somatic cells by means of chimera technologies. Results from many independent groups suggest that mouse and human iPS cells, once established, generally exhibit a normal karyotype, are transcriptionally and epigenetically similar to ES cells and maintain the potential to differentiate into derivatives of all germ layers. Injection of iPS cells into diploid (2n) blastocysts, similar to ES cells, frequently gives rise to high-contribution chimeras (mice that show major tissue contribution of the injected iPS cells in the host mouse), as has been shown by many different research groups[7,8]. A subset of these chimeras has successfully demonstrated germline contribution. However, only two reports so far have used the most stringent assay, that is, tetraploid embryo complementation[9,10].
Typically, genetic reprogramming of mouse and human somatic cells (iPS technology) can be achieved after ectopic expression of a defined combination of 4 transcription factors, namely c-Myc, Klf4, Oct4, and Sox2. It is known that c-Myc and Klf4 reprogramming factors are oncogenes and their expression or reactivation in iPS-derived mice causes tumors. The safety of iPS cell derivation can be improved by excluding c-Myc and Klf4 from the reprogramming cocktail or by selecting target cell types that already endogenously express these genes[11]. Recent studies provide a more efficient alternative that involves viral vector-free integration of reprogramming genes, followed by their removal. Recent adenoviral and plasmid-based strategies used in conjunction with latest generation transposon technology (e.g. PiggyBac and Sleeping Beauty transposons) may now potentially overcome some of these limitations[12-15].
The definition of “pluripotency” is that the cell can give rise to all three embryonic germ layers, i.e. mesoderm, endoderm, and ectoderm[16]. These three germ layers are the embryonic source of all cells of the body. The pluripotent nature of cells (either ES or iPS) is routinely tested by three methods. One test is to inject the cells into adult mice that are either genetically identical or are immune-deficient, so that the tissue will not be rejected. In the host animal, either when injected or when transplanted, these ES cells can become any cell in the body and form tumors called teratomas. A second test for pluripotency is to allow mouse ES cells to differentiate spontaneously in vitro, or to direct their differentiation along specific pathways. Within a few days after changing the culture conditions, ES cells aggregate and may form so-called embryoid bodies (EBs), further differentiating towards multiple cell lineages. Teratomas and EBs demonstrate that the ES cells are capable of developing into many cell types, derived from the three embryonic germ layers. Histological analysis has also demonstrated that iPS cells can give rise to teratomas comprising all three embryonic germ layers[17].
The third, in vivo method, is based on the capacity of cells to participate in the formation of germ cells when they are introduced into a preimplantational host embryo, resulting in the so-called “chimera mice”.
Chimera mice - or in brief “chimeras” - were first created in the 1960’s by Kristoph Tarkowski and Beatrice Mintz, by means of aggregating two eight-cell embryos, and were then produced by Richard Gardner and Ralph Brinster who injected cells into blastocysts. This revolutionary new technique opened up a new method for introducing any kind of cell (even genetically- modified) into the host embryo, thus creating a new chapter in mouse embryology, as well as in biotechnology.
The efficiency of producing live, transgenic and germline mice requires precise understanding of the mechanisms that could be vitally important for the maintenance of pluripotency, and therefore germline transmission of the ES cell lines. Despite successes in gene targeting in ES cells[18-20] during recent years, many factors that dramatically influence the efficiency of germline chimera mice generation have not been yet fully investigated.
One factor is the prolonged culture of cells. Once established and adapted to in vitro culture conditions, ES cells can be maintained for long periods of time. Stem cell derivation and maintenance imply extensive in vitro culture. This has raised the question of whether culture conditions could influence the developmental potential of stem cells and whether loss of germline capacity is due to the accumulated production of chromosome abnormalities and/or epigenetic alterations. In mouse and large animal models, extensive work has been performed on the epigenetic effects of in vitro culture. Indeed, recent work on ES cells has shown that stem-cell-derived tissues and embryos often fail to maintain stable epigenetic states, or the normal diploid karyotype. Several studies have reported that accumulation of epigenetic alterations, mostly in the imprinted genes, is the major cause of decreased or lost germline ability of ES cells. On the other hand, aneuploidy, rather than “loss of pluripotency”, in ES cells, is the one major cause of failure in obtaining contributions to all tissues of the adult chimera, including the germline. Euploidy is predictive for germline transmission and the karyotype analysis is crucial in any gene-targeting experiment.
Another factor is that, unfortunately, the founder mice are derived mostly from inbred strains, such as the C57BL/6 strain, which often shows poor viability or abnormalities due to developmental defects[2]. Still, the germline competency of the majority of ES cells (e.g DBA/1Ola, C3H/HeN, BALB/c, and FVB/N) is usually not comparable to the highly germline-competent 129 strains (129/Sv, 129/SvEv, and 129/Ola) derived ones[21,22]. Few ES cell lines are currently available from inbred strains (e.g. C57BL/6, BALB/c) and those have generally been produced with low success rates.
Another critical factor contributing to the success of germline-competent ES chimeras is the technique chosen to produce the chimeras. Attempts to improve the methods for generating ES mice chimeras were mainly carried out in order to establish ES cell lines with a higher potential for producing germline transmission. This strategy lead to the discovery that ES cell lines derived from hybrid mouse strains support the development of viable ES mice to a greater extent when compared with inbred ES cells[2] and significantly improved the technique. Although the effect of donor ES cells on the production of ES mice has been well studied, the technique still has a limitation in that ES mice can be generated only from specified ES cell lines. There are a number of options for generating chimeras from ES cells but each method has its advantages and disadvantages. In this review we will examine some of the conventional, and also the most recent methods for generating ES chimera mice, the advantages and the disadvantages of each method, and the factors that should be taken into account when deciding on one method in preference to another.
FACTORS INFLUENCING GERMLINE TRANSMISSION CAPACITY OF PLURIPOTENT CELLS
The efficiency of mouse ES cell germline transmission is strongly influenced by genetic background, and is maximized with ES cells that have spent a minimum amount of time in culture, that have a normal complement of chromosomes, and are not affected by epigenetic alterations. Here we give a brief account about how the factors mentioned above influence the germline transmission capacity and the developmental potential of mouse pluripotent stem cell lines.
Genetic background
It is still far from clear why certain strains are more amenable to ES cell derivation than others. In recent years, embryonic stem cells have been derived from various mouse strains. However, 129 ES cells (ES cell lines derived from different 129 backgrounds) are still widely used, partially due to poor germline transmission of ES cells derived from other strains. It is generally accepted that it is easier and more efficient to perform targeting for an ES cell line on a hybrid genetic background. A large number of inbred strains of mice exist, but only a small number are commonly used for establishing gene-targeted mice.
Genetic heterozygosity is presumed to be a crucial characteristic for postnatal survival of fully ES derived mice[23]. On the other hand, elimination of genetic background variability associated mostly with the use of 129 (129/Sv, 129/SvEv, and 129/Ola) embryonic stem (ES) cell lines, requires derivation of germline-competent ES cell lines from inbred mouse strains with specific genetic backgrounds, enabling generation of isogenic gene-targeted and control mice[24,25]. Mutagenesis by homologous recombination in ES cells[26] is an important means to the understanding of the molecular mechanisms of higher brain functions. This study requires gene targeting in embryonic stem (ES) cells derived from the strain suitable for brain function analysis and with a homogenous genetic background, such as the C57BL/6 strain. Auerbach et al[21] compared 129 and C57BL/6 ES cells and found that cells on C57BL/6 background are more sensitive to culture conditions and that it is more difficult to maintain them in culture than the 129 derived ones. Similar conclusions have also been reached by others[25].
Germline-competent ES cells have also been derived from other inbred strains, including C57BL/6, however, competency of germline transmission of these ES cell lines is not comparable to that of the 129 ES cells[20-24]. The developmental potential of C57BL/6 ES cells seems to be lost during cell culture in vitro[2], and seems to depend on several factors, such as the serum or even the feeder cells used for ES cell culturing. The quality of serum (even having the same catalogue number, but coming from different lots), pH of medium and the quality/origin of feeder layers used in different experiments can cause decreased developmental potential. Therefore care should be taken to introduce a broad variety of culture conditions in order to take ES cells germline. Mouse iPS cells are indistinguishable from embryonic stem (ES) cells in many respects and the production of germline-competent chimeras, and although this has not yet been studied, it is probable that it would also be influenced by the genetic background.
Some recent studies have described increased efficiency of derivation of germline- competent inbred ES cell lines, mostly by modifying current culture conditions[27,28] and have reported that using a culture medium conditioned by a rabbit fibroblast cell line and transduced with genomic rabbit leukemia inhibitory factor allows efficient derivation of ES cell lines from 10 inbred mouse strains (129/SvEv, 129/SvJ, C57BL/6N, C57BL/6JOla, CBA/CaOla, DBA/2N, DBA/1Ola, C3H/HeN, BALB/c, and FVB/N). Germline transmission was achieved by blastocyst injection of established ES cell lines after 10 or more passages from strains 129/SvJ, C57BL/6N, C57BL/6JOla, DBA/2N, DBA/1Ola, BALB/c and FVF/N. The efficiency of establishing ES cell lines and also generating germline chimeras from the C57BL/6 derived LK1 cell line was comparable with a widely used 129/SvJ derived GSI-1 ES cell line[28]. Sato et al[29] used leukemia inhibitory factor (LIF) and 6-bromoindirubin-30-oxime (BIO), a glycogen synthase kinase-3 (GSK3) inhibitor, and showed that BIO treatment significantly increased the expression levels of 364 genes including pluripotency markers such as Nanog and Klf family members. Chimeras derived from cell lines from LIF, BIO or GSK3 inhibitor- enriched medium were germline-competent. The current hope is that ES cell lines from “non-permissive” mouse strains will become more widely derivable, possibly by means of modifying ES cell culture conditions.
Chromosomal abnormalities
A key property of ES cells is that they maintain their euploid karyotype. This is crucial because a balanced diploid chromosome complement is necessary for proper meiosis.
The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimerism. Over the years, several studies have reported that chromosome make-up correlates with the capacity of ES cell clones to contribute to the formation of all tissues, including the germline, of the adult chimaeras. The data support the notion that karyological instability, and not loss of pluripotency, is the major reason for the lack of contribution to chimaeras of individual ES cell clones, and that karyotype analysis is a predictor of the germline transmission capacity of ES cell lines[30-34]. Some studies suggest that the long-term culture of iPS cells, similar to the situation for ES cells, has to be monitored carefully for culture-induced chromosomal abnormalities[35].
Other studies have also reported that the number of aneuploid mitoses in ES cells expands with increasing culture time[30,36] and that the ES cell clones with less than 50% euploid metaphases generated only a few and weak chimeras and non- germline[37]. It was shown that in particular, trisomy 8 is associated with a selective growth advantage in vitro and represents a common cause for the failure of ES cells to contribute to the germline[38,39]. Multicolor karyotyping technologies, including both multi-color fluorescence in situ hybridization (M-FISH) and spectral karyotyping (SKY), are recently developed molecular cytogenetic techniques for rapid visualization of genomic aberrations at sub-cellular level. Guo and colleagues[40], using the M-FISH method, recognized various chromosomal abnormalities in two independent ES cell lines: trisomy 8 in some mitoses, trisomy 14q and the deletion 6q in 100% of the cells studied[40]. The deletion 6q affected only a part of the respective chromosome and therefore the total number of 40 chromosomes was still retained. Some of these chromosomal abnormalities might be overlooked by standard G-banding analysis alone[41]. Presently, it is not known whether such translocations are detrimental to the achievement of high levels of chimerism or germline transfer. On the other hand, some studies have reported that the presence of chromosomal aberrations may reduce, but not necessarily eliminate, the ability of ES cells to contribute to normal development[42].
In summary, these data demonstrate a strong correlation between losing the germline-competence of ES cell lines and accumulation of chromosome abnormalities. However, research should aim to link specific components of the aberrant phenotypes with specific epigenetic alterations in gene expression.
Epigenetic alterations
Long term culture and in vitro manipulation of the ES cells can induce epigenetic alterations, which in turn can have long- lasting effects on the transcription patterns of the ES cell genome. Indeed, recent work on ES cells has shown that stem cell-derived tissues and embryos often fail to maintain stable epigenetic states, especially in imprinted genes[43-47]. So, any epigenetic changes caused by the number of passages would most probably affect the developmental pluripotency of ES cells and thus the viability of ES mice. Two further mouse studies have also investigated the epigenome of iPS cells on a larger scale. Maherali et al[17] used ChIP-Chip to investigate the presence of H3K4me3 and H3K27me3 in the promoter regions of 16 500 genes in one iPS cell line. Their results suggested that iPS cells were highly similar in their epigenetic state to ES cells with 94.4% of 957 “signature” genes (defined as genes that have a different epigenetic state between MEFs and ES cells) being reset to an ES-cell state in the respective iPS cell line[48].
In ES cells, the effects of methylation on expression of specific genes, particularly imprinted ones[43] and some retrotransposons[49], have been demonstrated in vivo.
Dean et al[43] investigated whether the prolonged culture of ES cells affects their pluripotency and whether it is associated with epigenetic alterations in imprinted genes. Two maternally expressed genes (Igf2r, H19) and two paternally expressed genes (Igf2, U2af1-rs1) were analyzed in ES cells, and in completely ES cell-derived fetuses. Altered allelic methylation patterns were detected in all four genes, and these were consistently associated with allelic changes in gene expression. It was also demonstrated that all methylation changes that had arisen in the ES cells persisted on in vivo differentiation to fetal stages. Alterations included loss of methylation with biallelic expression of U2af1-rs1, maternal methylation and predominantly maternal expression of Igf2, and biallelic methylation and expression of Igf2r. In most of the ES derived fetuses, the levels of H19 expression were strongly reduced, and the biallelic repression was associated with biallelic methylation of the H19 upstream region. ES fetuses derived from two of the four ES lines appeared developmentally compromised, with polyhydramnios, poor mandible development and interstitial bleeding and, in chimeric fetuses, the degree of chimerism correlated with increased fetal mass. This study created a model for how early embryonic epigenetic alterations in imprinted genes persist to later developmental stages, and are associated with aberrant phenotypes. Generation of pluripotent cells with correct epigenetic profile after reprogramming of somatic cells by the iPS technology is crucial for their developmental competence. It is yet to be demonstrated whether insufficient reprogramming in iPS cells would increase the probability of epigenetic alterations and subsequent developmental abnormalities in chimera embryos and fetuses.
Different studies have also reported that retrotransposon elements (REs) are transcribed during early mouse embryogenesis[50] and also in ES cell lines[51] and that transcriptional interference by active retrotransposons perturbs expression of neighboring genes in somatic cells, in a mosaic pattern corresponding to activity of each retrotransposon. Furthermore, the expression of REs also regulates host genes in preimplantation embryos[50]. Since ES cells are mostly isolated from the inner cell mass (ICM) of blastocysts, the expression of REs could be essential for in vitro and in vivo preservation of the genomic integrity and pluripotency of ES cells. Moreover, inadvertent alterations in the expression of two Res, i.e. intracisternal-A particle (IAP) and murine endogenous-retrovirus-L (MuERV-L), affected the pluripotency by losing the ability of germline transmission and started inducing the kinky tail phenotype in the chimera mice of high passage ES cell lines[50]. Therefore, the mechanism of epigenetic instability needs to be further explained and better understood, and consequently monitored when considering ES cells for transgenesis (chimera formation).
Es: mice chimera technologies
Tarkowski and Mintz made the first mouse embryonic chimeras by aggregating two eight-cell stage embryos. Since then, experimental manipulations have been modified in many different ways, for example, removing and/or reorienting cells, and adding them back at different stages. There are three commonly used methods for chimera production: (1) Diploid embryo (diploid embryo aggregation chimeras); (2) ES cells (diploid embryo aggregation and injection chimeras) (3) Diploid embryo (tetraploid embryo aggregation chimeras) and (4) ES cells (tetraploid embryo aggregation and injection chimeras). This section will focus on some of the conventional and also more recent methods for generating ES cell derived chimeras (ES chimera mice), the advantages and the disadvantages of each, and the factors that should be taken into account when one is chosen in preference to another.
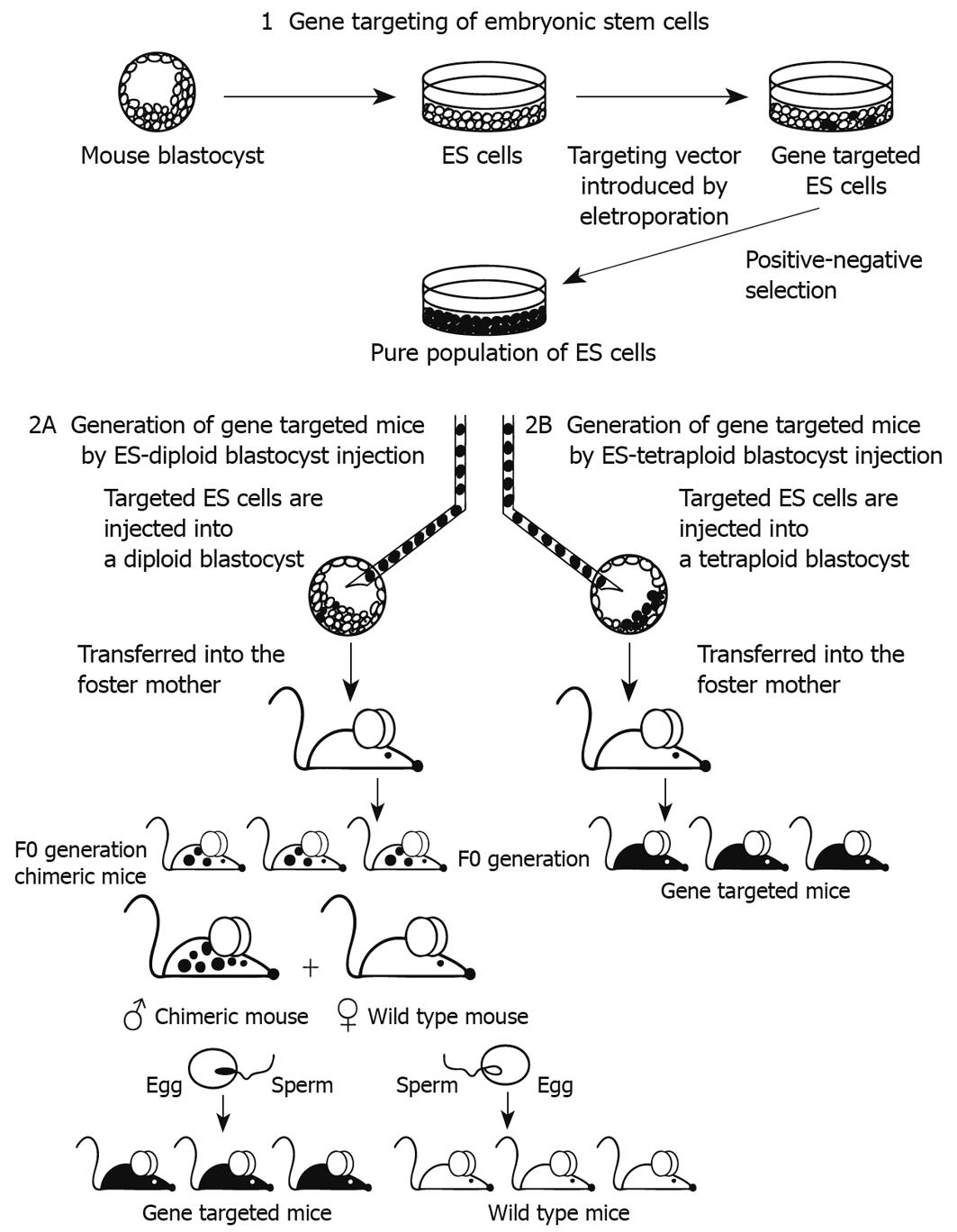
ES cells - diploid blastocyst injection chimeras: This technique was initially developed by Gardner[52,53] and used the introduction of the whole ICM into the blastocysts cavity (blastocoel).
Later on, conventional blastocyst injection and assisted piezo blastocyst injection[54] was extensively used to generate progeny from ES cells. These techniques involve the microinjection of 7-15 ES cells into the blastocoel. Contribution of donor ES cells to the germline of chimera mice allows the generation of mouse strains carrying the haplotype of ES cells. The chimeras are a mixture of cells derived from both donor ES cells and the recipient embryos. The determination of all tissues in the chimeras, including cells derived from the donor ES cells, is extremely difficult. Moreover, because of the developmental potential of diploid embryos prior to ES cells, they may restrain the pluripotency of ES cells in the chimeras[55]. In case of gene- targeted ES cell lines, the F0 chimera mice are only partially derived from the modified ES cells (Figure 1). If part of the germline is derived from the modified ES cells, these chimeras can be bred to obtain F1 generation mice that are uniformly heterozygous for the mutation of interest. Subsequent interbreeding of these heterozygous mice can result in F2 generation mice that are homozygous for the intended mutation. Because few mutant phenotypes can be detected in chimeric or heterozygous mutant mice, phenotyping requires derivation of homozygous mutant F2 mice. In addition, chimeras that are estimated to be 90% ES cell-derived based on coat color, can be inefficient germline transmitters, because coat color chimerism does not fully reflect ES cell contribution to internal organs (including germ cells).
Figure 1 General strategy for producing gene targeted mice by different Embryonic Stem (ES), chimera methods.
1: Gene targeting of ES cells, followed by selection of the ES cell clones containing the desired mutations; 2A: The ES cells are injected into diploid blastocysts. F0 chimera mice are only partially derived from the modified ES cells and are bred to obtain F1 generation mice that are uniformly heterozygous for the mutation of interest; 2B: The ES cells are injected into tetraploid blastocysts. The F0 generation is fully derived from the gene targeted ES cells.
In conclusion, the production of a mutant strain by using blastocyst injection method is a time-consuming task, often taking longer than 6-12 mo before the analysis of adult mutants can occur. It would be hoped that ES cell contribution is sufficient to enable germline transmission to result, with the transmission rates sufficient to enable heterozygote offspring to be obtained from 1st litters. Unfortunately, both the time and the number of mice generated to achieve that milestone are low. It remains a challenge to achieve good and reliable results, particularly with C57BL6 ES cells, where greater variation in outcomes is likely.
ES cells - tetraploid blastocyst injection chimeras: In the chimeras produced by injection of ES cells into tetraploid (4n) embryo[56-59], the tetraploid host embryo contributes to trophoblast lineage of the placenta and the extraembryonic endoderm[5] whereas the ES cells give rise to the mesoderm layer of the yolk sac, the amnions, the embryo proper and the allantois/umbilical cord. Using this strategy, live new-born mice can be generated that are completely derived from ES cells[2,3]. Embryo electrofusion and tetraploid blastocyst microinjection is a modification of the traditional ES cell-based method to generate targeted mutant mice (Figure 1). The tetraploidy is mostly induced by passing an electrical current across 2-cell embryos, resulting in a single 4n cell produced by the fusion of the two 2n blastomeres[60,61].
The tetraploid method is limited by a number of factors, and its success appears to be highly variable, depending on host embryo blastocyst strain, ES cell strain, ES cell line passage number, and the quality of in vitro cell preparation. Most ES cells used to date for tetraploid blastocyst injection are of 129 mouse background strain or F1 hybrid ES cells (C57BL/6 × 129)[62]. The use of either pure 129 or C57BL/6 ES cells for tetraploid blastocyst microinjection is feasible[24,25] but to date F1 ES cells have proven to be more robust[57].
Viability of embryos from tetraploid injections is reportedly lower than with diploid embryos, with considerable strain variation[58] In addition, in one study, outbred Swiss Webster blastocysts exhibit greater developmental potential with the tetraploid technique than do blastocysts from 4n B6CBAF2 hybrid mice[57]. Post-implantation Swiss 4n embryos were observed more frequently and were more likely to develop advanced embryonic structures than 4n B6CBAF2 embryos in 4n:2n chimeras. The data show that the 4n component can persist at gastrulation and into midgestation in 4n:2n chimeras and that at later stages these 4n cells may colonize tissue sporadically throughout the embryo. The mechanism behind this difference in developmental potential is most likely explained by the presence of classes of alleles that promote or inhibit a cell’s ability to regulate a duplicated genome.
A more recent retrospective study proved that outbred and hybrid tetraploid host embryos are more efficient for tetraploid complementation assay than inbred strains[23]. The reason could be that embryos used in the tetraploid procedure must not only survive in vitro for 3 to 4 d, but also withstand the additional electrofusion manipulation. Diminished ability of embryos to tolerate the additional manipulations would be expected with inbreeding depression. It was also shown that the use of 3 × 4n host embryos for aggregation with ES cells is more effective for generating ES mice than using 1 × 4n host embryo[63].
Another recent study reported the generation of several iPS cell lines that are capable of generating viable, live-born progeny by tetraploid complementation[9,10]. Therefore, even if the tetraploid method is limited by a number of factors, it has proven to be one of the most commonly used for mice generations fully derived from normal ES, gene targeted ES or even iPS cells.
ES cells - diploid eight-cell stage embryo injection chimeras: Interest in the ES cell injection into pre-blastocyst stage embryos was reawakened with a publication in 2007 from the US Company Regeneron[64]. Their “VelociMouse” methodology uses laser-assisted injection of ES cells into eight cell-stage host embryos, and generates fully ES cell -derived mice by an easier, more practical means from a variety of ES cell backgrounds. Further work in response to this publication has shown successful generation of fully ES cell- derived mice through the use of piezo injection[65] or through the use of standard beveled needles[66].
It was reported that F0 generation mice are able to efficiently transmit the mutation through the germline; they are fully derived from the modified ES cells and permit immediate phenotyping. The host contamination does not exceed 0.1% and demonstrates that the phenotypes of these and the eight-cell method is effective for either inbred ES cells, like C57BL/6 and 129, or hybrid ES cells[65].
The new methods were reported to be easier and more efficient than the tetraploid complementation method. On the other hand, these methods require expensive equipment and extensive experience, and demand more time than the conventional system, which may influence the quality of the micromanipulated embryos. In addition, the success of generation mice fully derived from ES cells could be, similar to the tetraploid complementation assay, highly variable. Factors like prolonged culturing, their genetic background, chromosomal abnormalities and/or the epigenetic profile of the ES cells could lead to a compromised developmental potential for high rate ES cells-derived fetuses.
CONCLUSION
In mice, ES cell lines can vary considerably in their germline transmission capacity and their developmental potential. The efficient production of live, transgenic and germline mice requires precise understanding of the essential mechanisms for the maintenance of pluripotency, and therefore germline transmission, of the ES cell lines. Retention of germline competence may be due, at least in part, to ES cell line genetic background, a reliable epigenetic profile, euploid karyotype and, last but not least, the most appropriate chimera method. Use of iPS cell lines for chimera production presents new challenges for investigators. A significant amount of further investigation must be undertaken to explore and understand the precise relationships between all the above factors in order to build appropriate mouse models and to develop therapeutic strategies for a variety of diseases. Continuous research on ES cells remains crucial in order to validate iPS cells and to determine which cells would be most useful for specific purposes.
This review is focused on the germline chimera- forming potential of mouse ES and iPS cells. On the other hand, these findings might provide valuable insights for human cell therapy perspectives, but further clarification is needed on how ES and iPS cells differ in terms of biology, action mechanisms, and curative potential. The world will be watching these experiments to see if the field can live up to its promises. Also, for a better public understanding of science, the ethicists and non-specialists will need clear information regarding the basics of stem cell biology, and the predictive power of animal models.