Copyright
©The Author(s) 2017.
World J Stem Cells. Aug 26, 2017; 9(8): 133-143
Published online Aug 26, 2017. doi: 10.4252/wjsc.v9.i8.133
Published online Aug 26, 2017. doi: 10.4252/wjsc.v9.i8.133
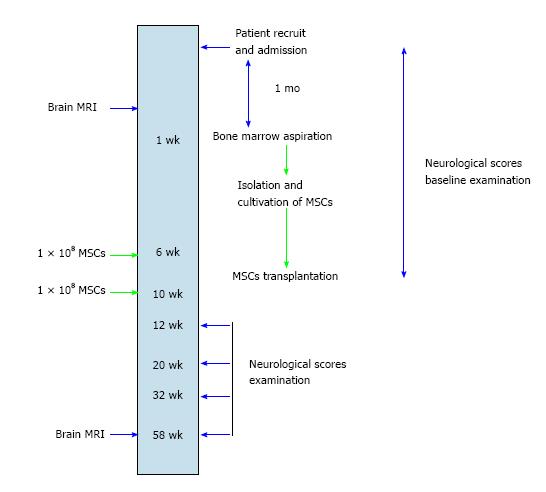
Figure 1 Workup schema for patients recruited to the clinical trial of autologous mesenchymal stem cell therapy.
MSC: Mesenchymal stem cell; MRI: Magnetic resonance imaging.
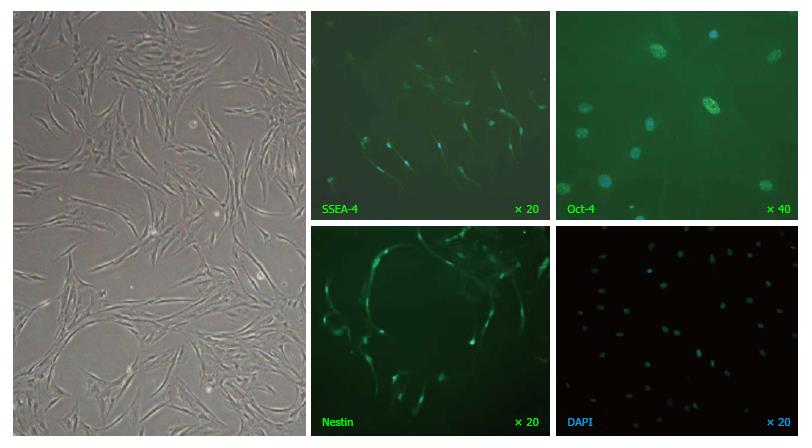
Figure 2 A representative image of mesenchymal stem cells at passage-8 captured under phase-contrast microscopy (left panel) and immunofluorescence staining of stage-specific embryonic antigen SSEA-4, transcription factor Oct-4 and neural stem cell marker Nestin (green fluorescence) with nuclei counterstained by DAPI (blue fluorescence).
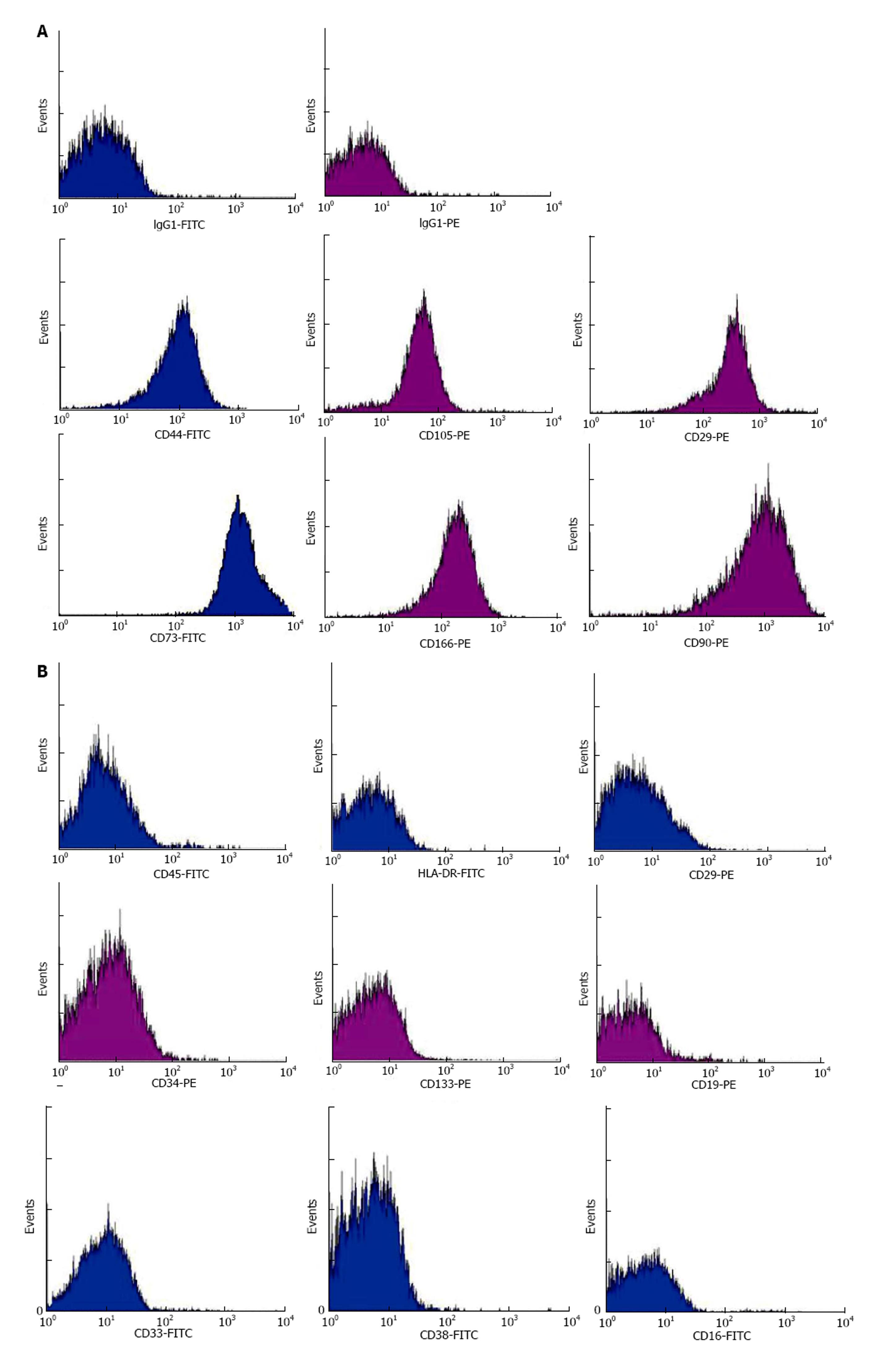
Figure 3 Representative histograms derived from infusates by flow cytometric analyses of mesenchymal stem cell markers CD29, CD44, CD73, CD90, CD105 and CD166 (A) and haemic markers HLA-DR, CD45, CD3, CD19, CD16, CD33, CD38, CD34 and CD133 (B).
FITC conjugation in blue and PE conjugation in purple.
- Citation: Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, Wong KSL, Ng HK, Poon WS. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells 2017; 9(8): 133-143
- URL: https://www.wjgnet.com/1948-0210/full/v9/i8/133.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i8.133