Copyright
©The Author(s) 2015.
World J Stem Cells. Apr 26, 2015; 7(3): 605-617
Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.605
Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.605
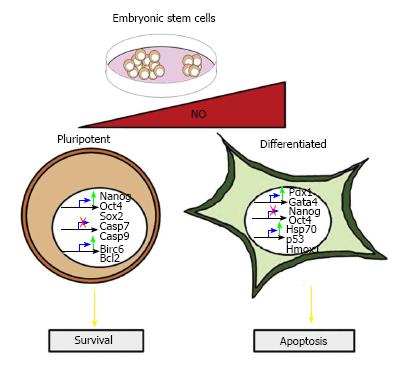
Figure 1 The dual roles of nitric oxide in stem cell pluripotency and apoptosis.
Low amounts of nitric oxide (NO) induce the expression of Nanog, Oct4 and Sox2. This confers protection from apoptosis by down-regulating pro-apoptotic genes including Casp7, Casp9, as well as up-regulating the anti-apoptotic genes Bcl-2 and Birc6. High NO concentrations promote apoptosis markers, such as Poly (ADP-ribose) polymerase degradation, cleaved caspase-3, and increased p53 protein levels. Cells expressing Hsp70, a cyto-protective gene, are resistant to oxidative stress and enter into a differentiation program. Differentiation events are initiated by the down-regulation of Nanog and Oct4 and the expression of definitive endoderm markers, such as Pdx1 and Gata4.
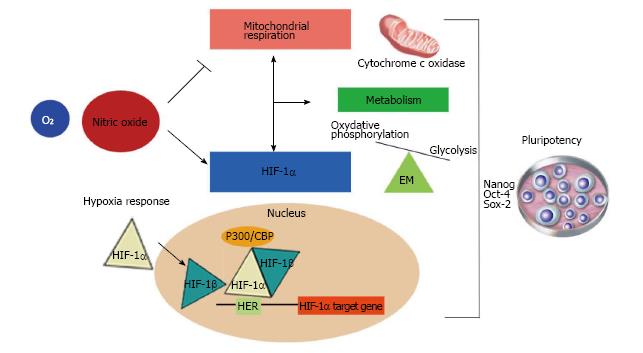
Figure 2 The role of nitric oxide as a regulator of mitochondrial function, metabolism and the hypoxia response.
The wide range of functions controlled by nitric oxide (NO) is a consequence of its dose-dependent dual roles. Physiological concentrations of NO inhibit cytochrome c oxidase in a reversible manner in competition with O2. This interaction depends on NO and oxygen concentrations and the redox state of the enzyme. Because of this, it has been reported that NO might be a regulator of cell respiration, modulating metabolic changes responsible for an increase in glycolytic pathway flux to maintain energy homeostasis. On the other hand, NO can also regulate the hypoxia response through hypoxia inducible factor-1α (HIF-1α). In a normoxic microenvironment, NO induces HIF-1α accumulation in a mitochondrial-dependent and -independent manner. Hypoxia conditions promote delayed cell differentiation. These facts indicate that NO might maintain pluripotency via regulation of metabolism and the hypoxia response. EM: Energy metabolism.
- Citation: Beltran-Povea A, Caballano-Infantes E, Salguero-Aranda C, Martín F, Soria B, Bedoya FJ, Tejedo JR, Cahuana GM. Role of nitric oxide in the maintenance of pluripotency and regulation of the hypoxia response in stem cells. World J Stem Cells 2015; 7(3): 605-617
- URL: https://www.wjgnet.com/1948-0210/full/v7/i3/605.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i3.605