Copyright
©The Author(s) 2015.
World J Stem Cells. Mar 26, 2015; 7(2): 418-427
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.418
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.418
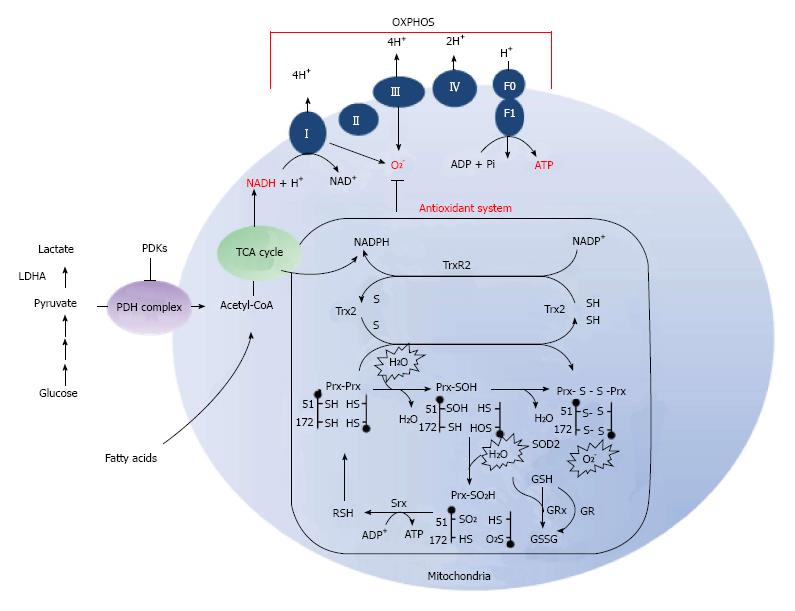
Figure 1 Antioxidant and oxidative phosphorylation systems in mitochondria.
Under normal conditions, normal cells rely primarily on oxidative phosphorylation for ATP synthesis, whereas cancer cells rely more on glycolysis. Pyruvate from glycolysis is converted to acetyl-CoA, CO2, and NADH by pyruvate dehydrogenase (PDH). Acetyl-CoA enters the TCA cycle by the citrate synthase-mediated reaction with oxaloacetate to generate citrate. NADH is oxidized first by Complex I in the electron transport chain (OXPHOS). Electrons from Complex I and II are transferred to coenzyme Q10, then passed on to Complex III, cytochrome c, Complex IV, and finally to O2 to generate H2O. O2- is converted to H2O2 through the action of SOD2 and/or spontaneous dismutation. H2O2 is eliminated by three mechanisms: (1) glutathione (GSH) peroxidase (GPx) coupled to GSH and GSH reductase (GR); (2) Prx3 coupled to Trx2 and Trx reductase (TrxR) 2; and (3) non-enzymatic eliminating by redox compounds. The H2O2 selectively oxidized cysteine Cys-SH to Cys-SOH, which then reacts with the resolving cysteine Cys-SH of the other subunit in the homodimer to form an intermolecular disulfide bond. The disulfide bond is reduced by Trx2. Moreover, the generated Cys-SOH is oxidized to Cys-SO2H. Reactivation of the enzyme is achieved by reduction of the Cys-SO2H moiety and is catalyzed by sulfiredoxin (Srx). Nicotinamide adenine dinucleotide phosphate (NADPH) is utilized by the reductases in the peroxidase system (GR and TrxR) to reduce disulfide bonds formed in proteins during the elimination of H2O2. TCA: Tricarboxylic acid; ATP: Adenosine triphosphatase; ADP: Adenosine diphosphate.
- Citation: Song IS, Jeong JY, Jeong SH, Kim HK, Ko KS, Rhee BD, Kim N, Han J. Mitochondria as therapeutic targets for cancer stem cells. World J Stem Cells 2015; 7(2): 418-427
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/418.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.418