Copyright
©The Author(s) 2015.
World J Stem Cells. Mar 26, 2015; 7(2): 266-280
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.266
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.266
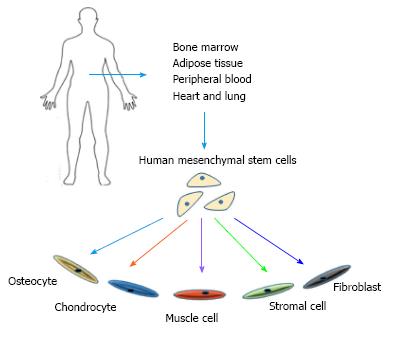
Figure 1 Potentials and sources of mesenchymal stem cells.
Mesenchymal stem cells can be collected from various sources within human body and have the ability to differentiate into a variety of lineages.
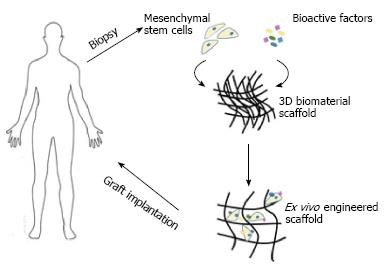
Figure 2 Overview of tissue engineering strategy of incorporating scaffolds with mesenchymal stem cells.
Mesenchymal stem cells (derived directly from the patient) are expanded in the laboratory, whereby the necessary environment for their growth has been prepared. These cells are then seeded onto a scaffold and either allowed to differentiate ex vivo pre-implantation or the scaffold is immediately implanted.
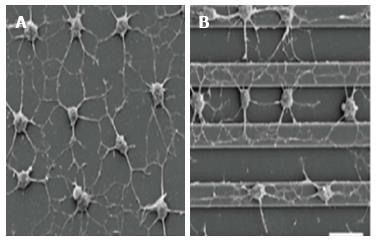
Figure 3 Comparison of different topography strategies employed to investigate the effects of anisotropic vs isotropic cytoskeletal tension on cultured mesenchymal stem cells.
(A) nonpatterned substrates caused randomly oriented cell protrusions to be formed, while (B) alignment of elaborated processes in the direction of the grooves were induced by micropatterned surfaces, mimicking the native structure and orientation of the natural extracellular matrix proteins[52].
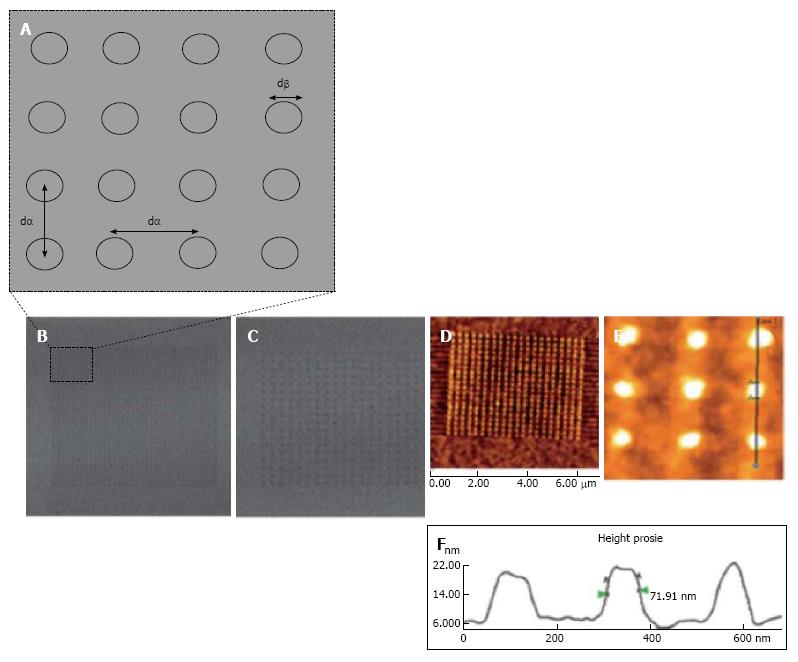
Figure 4 Nanopatterned gold surfaces examination for the effect of both the nanotopography and terminating chemical functionality.
A: Nanopatterned surfaces used for mesenchymal stem cell control and differentiation exhibiting dot to dot pitch (dα) and dot diameter (dβ); B: Lateral Force Microscopy (LFM) image of small area 280 nm pitch array; C: LFM image of 140 nm pitch array; D-F: Atomic force microscopy topographical image of an alkanethiol resist array fabricated on gold surface following chemical etching. An average diameter feature (dβ) of 70 nm was shown on the cursor profile[49].
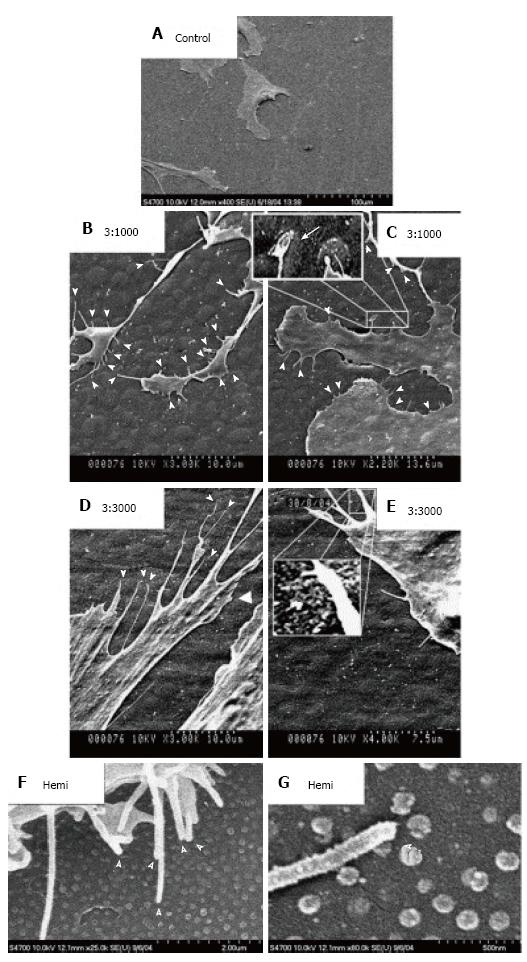
Figure 5 Scanning electron micrographs of human mesenchymal stem cells cultured on control and test materials.
A: On planar control materials cells showed normal morphologies; B, C: Filopodial of the human mesenchymal stem cells (hMSCs) interacts with the 3:1000 substrates (arrowheads); C: hMSC filopodia are curving around an island; D, E: Filopodial interactions with the 3:3000 substrates (arrowheads); E: A filopodia curving around an island is clearly observed; F: filopodial interactions with the hemi substrates (arrowheads); G: Filopodia curving around a hemisphere (arrowhead)[65].
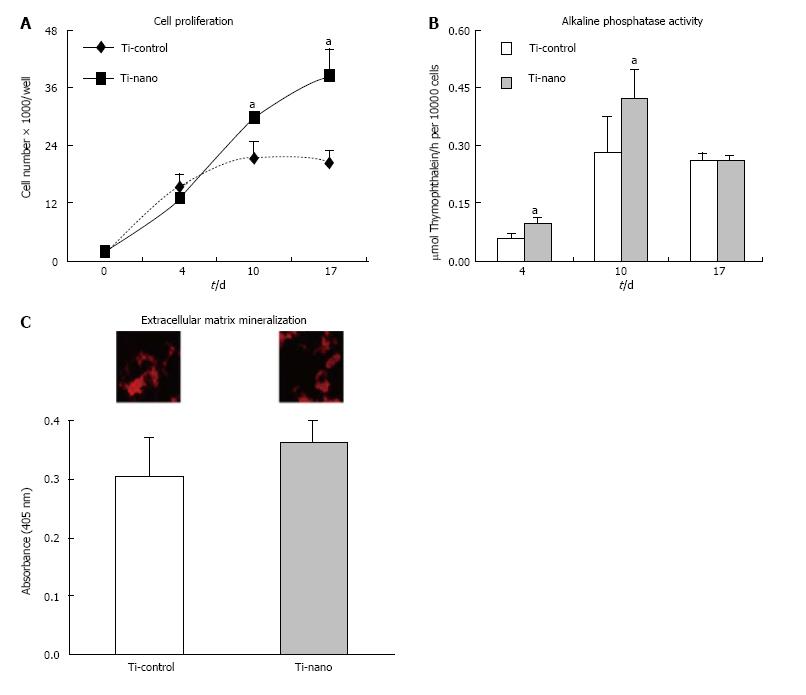
Figure 6 Investigation of the effects of nanotopography on proliferation.
(A), alkaline phosphatase (Alp) activity (B), and extracellular matrix mineralization (C) of mesenchymal stem cells differentiated into osteoblasts and cultured on nanotopography in an osteogenic medium compared to control Ti surfaces. A: The number of cells was significantly increased on Ti with nanotopography on days 10 (P = 0.07) and 17 (P = 0.03); B: Higher Alp activity was supported by Ti surface with nanotopography supported on days 4 (P = 0.01) and 10 (P = 0.04); C: The difference in the level of calcium mineralisation in the matrix (insets) was not statistically significant (P = 0.13) by comparing both surfaces. The data are presented as mean ± standard deviation (n = 4). aIndicates statistically significant difference[66].
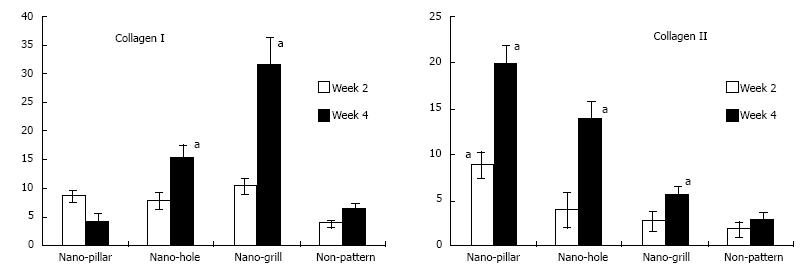
Figure 7 The effect of nano-patterned surfaces on the expression of cartilaginous genes.
Real time polymerase chain reaction was used to analyse mRNA expression levels of cartilaginous genes at week 2, 4 or 6 of chondrogenic differentiation, which was normalised to their respective glyceraldehyde-3-phosphate dehydrogenase expression and expressed as fold changes relative to undifferentiated mesenchymal stem cells. n = 3 per group, mean ± SD. aP < 0.05 which was considered statistically significant compared to non-patterned surface[55].
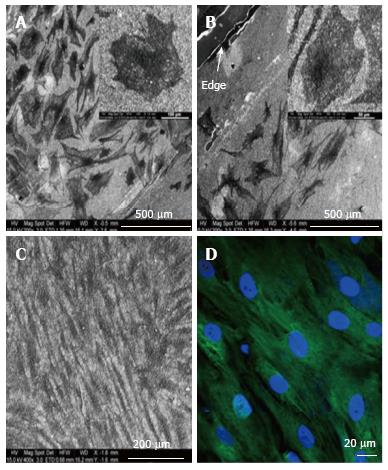
Figure 8 Electron microscopy images of differentiated and undifferentiated mesenchymal stem cells on Coll/PLLCL nanostructured nanofibrers.
A: Mesenchymal stem cell (MSC) directed to differentiate along the epidermal lineage when cultured in epidermal induction medium; B: Epidermally differentiated MSC on the edge of a Coll/PLLCL scaffold (as shown by arrow); C: Electrospun Coll/PLLCL nanofibers seeded with undifferentiated MSC cultured in normal growth medium; D: MSC grown in normal growth medium on Coll/PLLCL nanofibers stained with Ker 10, after 15 d cell culture, imaged using laser scanning confocal microscope[85].
- Citation: Salmasi S, Kalaskar DM, Yoon WW, Blunn GW, Seifalian AM. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J Stem Cells 2015; 7(2): 266-280
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/266.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.266