Copyright
©2014 Baishideng Publishing Group Inc.
World J Stem Cells. Jul 26, 2014; 6(3): 278-287
Published online Jul 26, 2014. doi: 10.4252/wjsc.v6.i3.278
Published online Jul 26, 2014. doi: 10.4252/wjsc.v6.i3.278
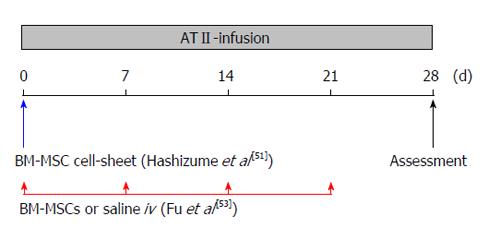
Figure 1 Diagram of bone marrow-derived mesenchymal stem cell cell-sheet implantation or bone marrow-derived mesenchymal stem cell intravenous-administration protocol.
At the time of Alzet osmotic minipump implantation, the BM-MSC cell-sheet was implanted into the nearby abdominal aortic adventitia[51], and 1 × 106 BM-MSCs (in 0.2 mL saline) or 0.2 mL saline were injected intravenously via the tail vein every week[53]. Mice were sacrificed and assessed on day 28. ATII: Angiotensin II; iv: Intravenous; BM-MSC: Bone marrow-derived mesenchymal stem cell.
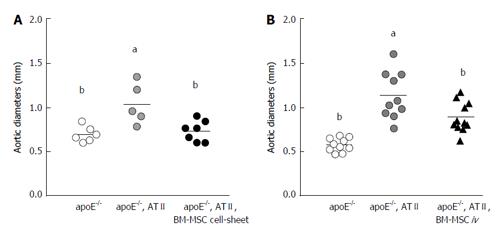
Figure 2 Bone marrow-derived mesenchymal stem cell cell-sheet implantation or bone marrow-derived mesenchymal stem cell IV-administration attenuates aortic aneurysm progression and expansion.
Aortic diameter was measured at the infrarenal aorta in the bone marrow-derived mesenchymal stem cell (BM-MSC) cell-sheet (A) or the BM-MSC IV-administration. Data are assessed by one-way ANOVA with Bonferroni correction. aP < 0.05 vs apoE-/- group, cP < 0.05 vs apoE-/- + ATII group. Data are from Hashizume et al[51] and Fu et al[53]. ATII: Angiotensin II; iv: Intravenous.
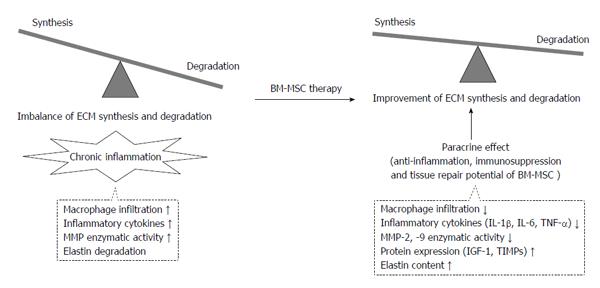
Figure 3 Attenuation of aortic aneurysm development and growth is associated with improvement of the imbalance between degradation and synthesis of extracellular matrices by bone marrow-derived mesenchymal stem cell therapy.
ECM: Extracellular matrices; BM-MSC: Bone marrow-derived mesenchymal stem cell; TIMP: Tissue inhibitor of metalloproteinase; MMP: Matrix metalloproteinase; IL: Interleukin; IGF: Insulin-like growth factor.
- Citation: Yamawaki-Ogata A, Hashizume R, Fu XM, Usui A, Narita Y. Mesenchymal stem cells for treatment of aortic aneurysms. World J Stem Cells 2014; 6(3): 278-287
- URL: https://www.wjgnet.com/1948-0210/full/v6/i3/278.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i3.278