Copyright
©2014 Baishideng Publishing Group Co.
World J Stem Cells. Apr 26, 2014; 6(2): 134-143
Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.134
Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.134
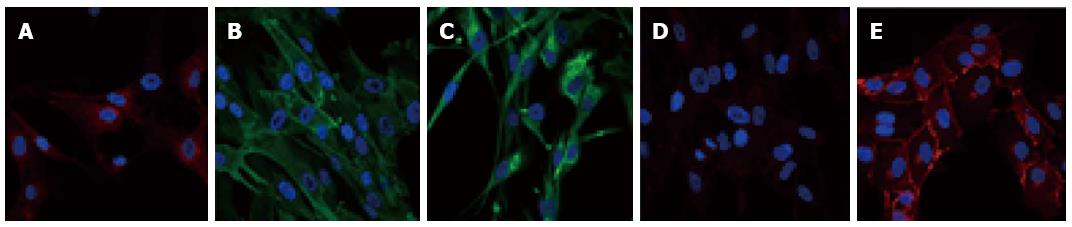
Figure 1 Pericyte immunophenotyping.
Pericytes express antigens allowing their identification. However, there is currently no specific marker to identify them. Therefore, to distinguish pericytes from other cell types, both positive and negative markers are used. For example, pericytes are known to be positive for platelet-derived growth factor receptor β (PDGFR-β)/CD140b (A), Alanine aminopeptidase N/CD13 (B), and for the stem cell protein nestin (C). Pericytes are also negative for VE-Cad/CD144 (D) that is detected in human brain endothelial cells (E). Specific antigenic labeling is in green or red and nuclei are 4’,6-diamidino-2-phenylindole stained (blue).
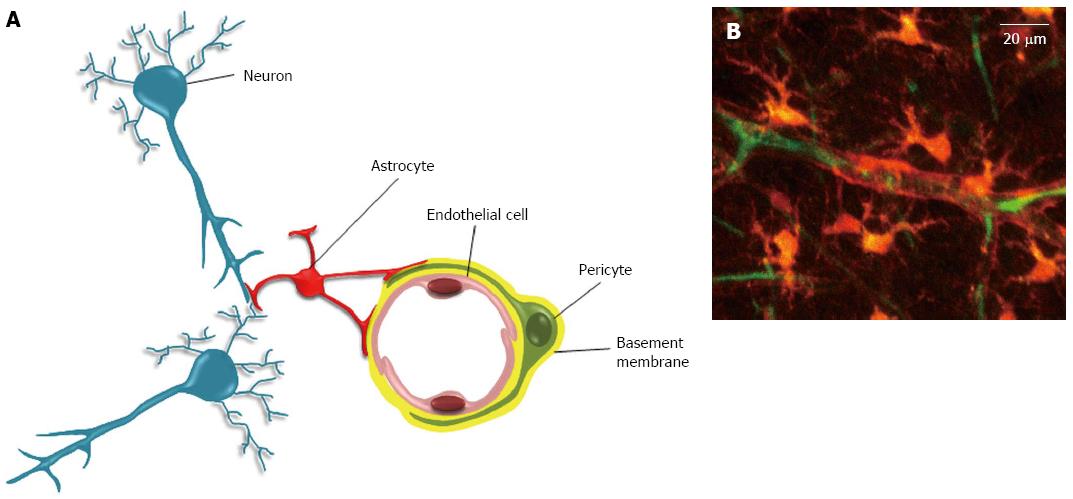
Figure 2 The neurovascular unit.
A: The neurovascular unit. In the neurovascular unit, pericytes are located on the abluminal side of endothelial cells (EC). Both cells are ensheathed by the basement membrane (BM). The covering of EC by pericytes is incomplete, and interruptions in BM can allow direct contacts between pericyte and EC. These contacts occur through peg and socket structures, and adherent and gap junctions (not shown)[59]. The abluminal side of the basement membrane is also contacted by astrocytes endfeet. In addition to these cells, the neurovascular unit also includes neurons, and microglial cells (not shown); B: Two-photon microscopy of a neurovascular unit. Following injection in the rat tail vein, the sulforhodamine-B dye crosses the blood brain barrier and stains astrocytes and pericytes in orange (reproduced from[115]). The blood plasma is shown in green after iv injection of FITC-dextran (Mw 70 kDa). Neurons, endothelial and microglial cells are not shown here.
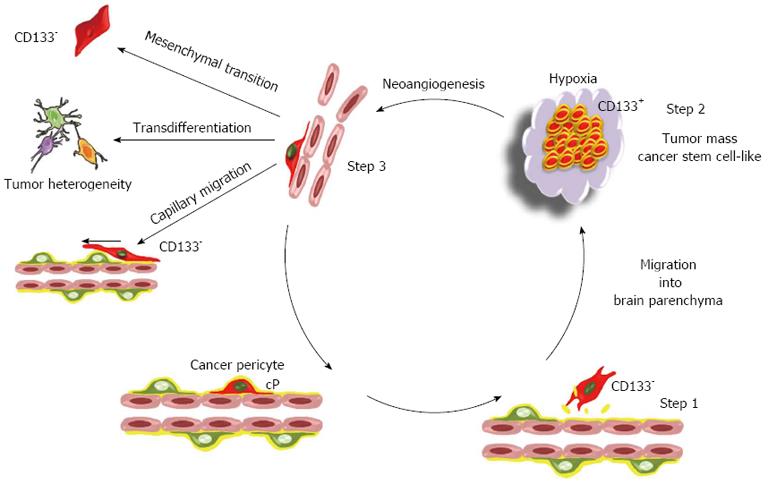
Figure 3 The cancer pericyte model: A perpetuum mobile.
The proposed model of brain tumor is based on the mesenchymal stem cell potential of pericytes. In this model, the brain cancer initiating cell is a cancer pericyte (cP) harbouring oncogenic alterations and located on a brain capillary. After disruption of the basement membrane by proteases, it detaches from the vessel wall and migrates into brain parenchyma as normal pericytes do following injury[76] (step 1). During the passage from a vascular to a neural environment the pericyte acquires a CD133+ neural stem cell-like phenotype, as observed in vitro for non-transformed pericytes[72]. Such a transition towards a neural stem cell phenotype is already observed for non-transformed pericytes at least in vitro. This generates the CD133+ cancer stem cell pool (step 2). Amplification of the cancer stem cell pool generates hypoxia that triggers neoangiogenesis and the migration of endothelial cells towards the lesion as well as the migration of cancer stem cells towards endothelial capillaries[83] (step 3). Cancer stem cells within this new vascular microenvironment reacquire a CD133- pericyte-like phenotype. At this stage, they can either integrate into the tumor neovasculature and reinitiate a new cycle generating a perpetuum mobile, or migrate along capillaries and invade brain as previously described[116,117]. Alternatively, due to the mesenchymal stem cell potential of pericytes, these pericyte-like cancer cells can acquire mesenchymal traits and progress towards a more aggressive mesenchymal phenotype. The transdifferentiation potential of pericyte-like cancer cells could in turn participate to the cellular heterogeneity found in glioblastoma multiforme. Since CD133 is not detected in pericytes, the existence of CD133- pericyte-like cancer stem cells provides an issue to the controversy regarding the existence in glioma tumors of both CD133+ and CD133- cancer stem cells[118,119]. Note that this model is not exclusive. The transformation of a glial or neural stem cell might also generate cancer initiating cells.
- Citation: Appaix F, Nissou MF, Sanden BVD, Dreyfus M, Berger F, Issartel JP, Wion D. Brain mesenchymal stem cells: The other stem cells of the brain? World J Stem Cells 2014; 6(2): 134-143
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/134.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.134