Copyright
©2013 Baishideng Publishing Group Co.
World J Stem Cells. Oct 26, 2013; 5(4): 217-228
Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.217
Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.217
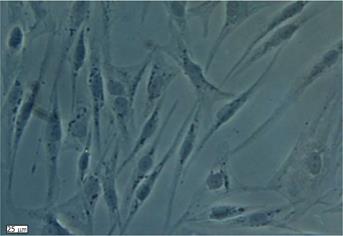
Figure 1 In vitro morphology of human adipose derived stem cells after collagenase digestion and plastic adherence selection.
In the primary human adipose tissue derived stem cell culture, cells exhibited a spindle-shaped fibroblastic morphology in colonies after 7-10 d.
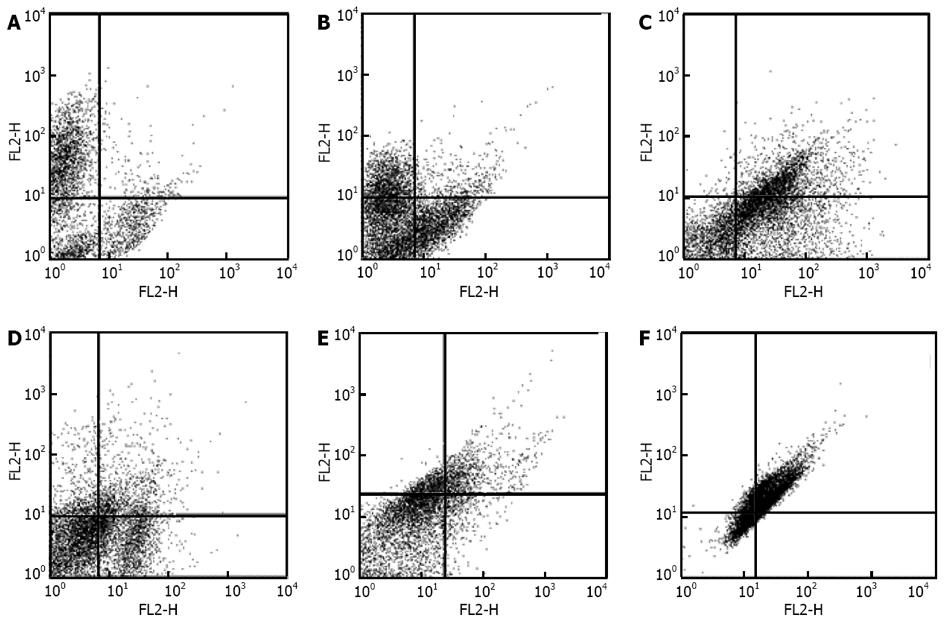
Figure 2 Characterization of human adipose tissue derived stem cells by flow cytometry.
Human adipose tissue derived stem cells in third passage were tested against the following antibodies and analyzed by FACS Caliber. A: CD45-FITC/CD34-PE; B: CD31-FITC/CD73-PE; C: CD90-FITC/CD105-PE; D: CD16-FITC/CD29-PE; E: CD44PE/ CD106-PEcy5; F: CD14-PE/CD55-PE5. MSCs were negative for CD31, CD34 and CD45 and positive for CD44, CD73, CD90, CD105, CD29, CD106 and CD55.
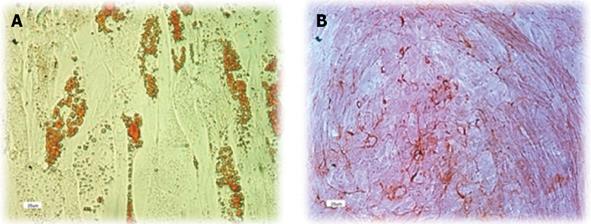
Figure 3 Multi lineage differentiation ability of human adipose tissue derived stem cells.
Human adipose tissue derived stem cells of 3rd passage were cultured in specific differentiation mediums for 14-21 d. A: Oil Red O staining shows the presence of fat vacuoles in adipocytes; B: Differentiation into the osteocyte lineage was shown by staining with Alizarin.
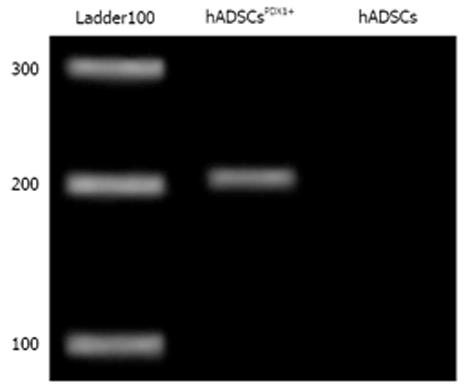
Figure 4 Real-time reverse transcription-polymerase chain reaction of PDX1 gene from puromycin resistance human adipose tissue derived stem cellsPDX1+.
Expression of PDX1 gene in transduced human adipose tissue derived stem cells (hADSCs) was confirmed by amplification of a 201 bp fragment compared to no amplification in control hADSCs.
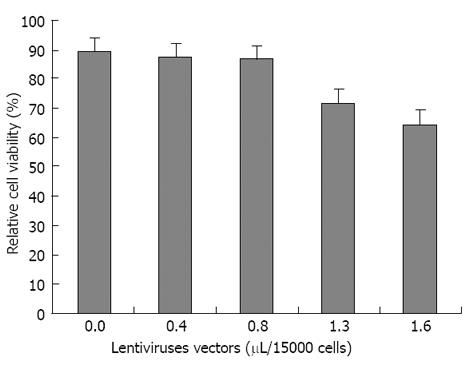
Figure 5 Relative cell viability of transduced human adipose tissue derived stem cells compared to untransduced human adipose tissue derived stem cells determined by MTT assay.
In the horizontal axis, the first value 0.0 is related to untransduced human adipose tissue derived stem cells (hADSCs) without any virus transduction. The optimum volume of non-integrated lentiviruses harboring PDX1 was obtained as 0.8 μL/15000 hADSCs that has been used for cell transductions.
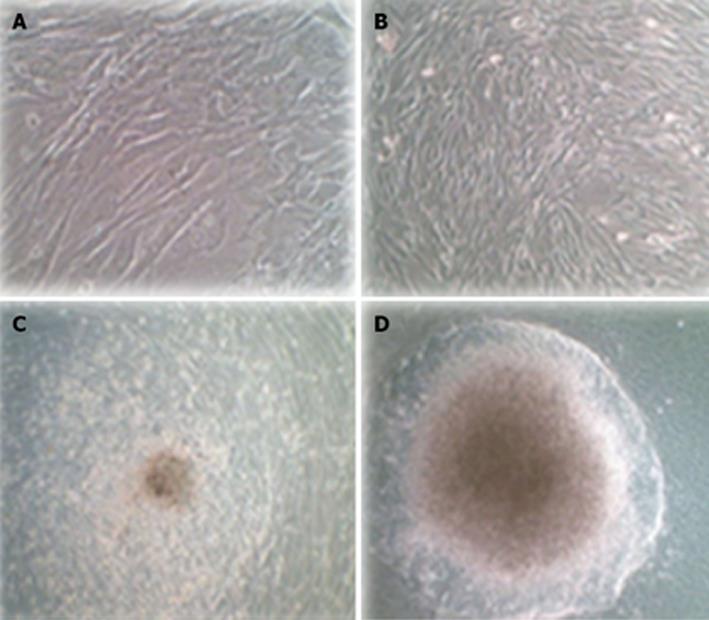
Figure 6 Morphological differentiation of human adipose tissue derived stem cellsPDX1+ three weeks after second transduction cultured in differentiation medium containing high glucose Dulbecco’s modified Eagle’s medium supplement by B27, nicotinamide and βFGF.
A: Untransduced human adipose tissue derived stem cells (hADSCs) were a typically adherent spindle shape; B: Human ADSCsPDX1+ became oval-type cells after second transduction; C: Human ADSCsPDX1+ formed clusters in the medium containing high glucose Dulbecco’s modified Eagle’s medium (DMEM), B27 and nicotinamide 7 d after transduction; D: Human ADSCsPDX1+ continued to differentiate and formed larger clusters in 3 wk in the medium containing high glucose DMEM, B27, nicotinamide and βFGF.
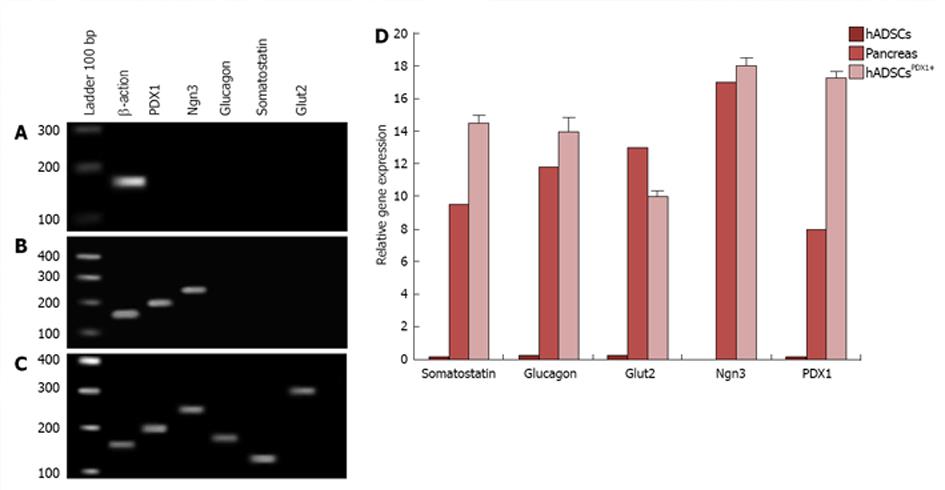
Figure 7 Experiment of pancreatic marker genes consisting of Glut2, PDX-1, Ngn3, somatostatin and glucagon in human adipose tissue derived stem cellsPDX1+ compared to human adipose tissue derived stem cells (negative control) and human pancreatic tissue (positive control) by real time PCR.
A: In human adipose tissue derived stem cells (hADSCs), no tested pancreatic markers were amplified except Β-Actin as internal real-time reverse transcription-polymerase chain reaction positive control; B: Expressions of PDX-1 and Ngn3 markers in hADSCsPDX1+, 7 d after second viral transduction; C: Expressions of PDX1, Ngn3, glucagon, somatostatin and Glut2 markers in hADSCsPDX1+, 21 d after second viral transduction indicating for beta like cell differentiation; D: Relative gene expression of PDX1, Ngn3, glucagon, somatostatin and Glut2 was mostly increased in hADSCsPDX1+ compared to human pancreatic tissue on the 21st day after second viral transduction. All PCR products were verified by electrophoresis and sequencing and relative gene expression data were analyzed using 2-∆∆Ct method.
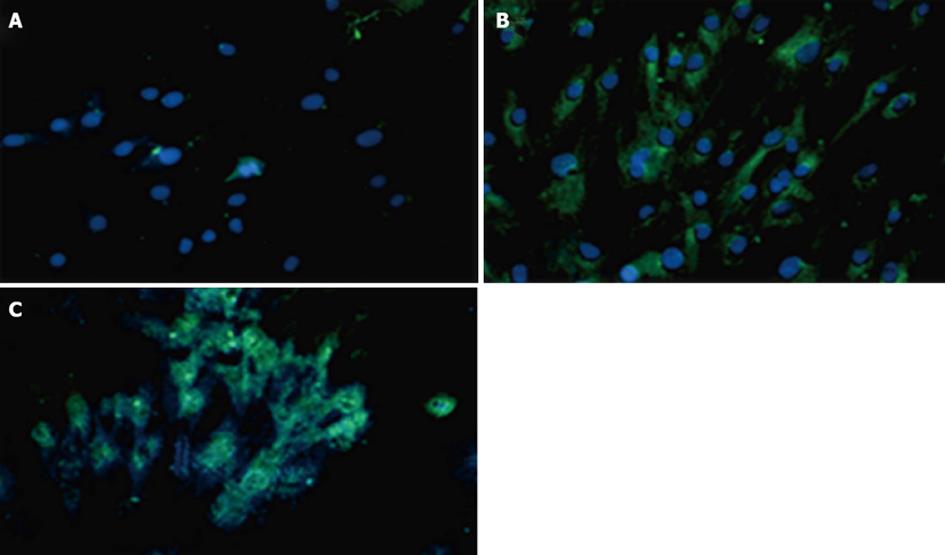
Figure 8 The immunocytochemistry experiments using PDX1 and insulin antibodies 7 d after transduction of human adipose tissue derived stem cells with non-integrated lentiviruses harboring PDX1 and selection of transduced cells with puromycin.
FITC conjugated PDX1 antibody (green color) and FITC conjugated insulin antibody (green color) were used for immune staining of PDX1 and insulin proteins and DAPI (blue color) was used for nuclear staining. A: Untransduced human adipose tissue derived stem cells (hADSCs) represented no green signals; B: hADSCsPDX1+ represented green signals related to insulin expression in the cytoplasm; C: hADSCsPDX1+ represented green signals related to PDX1 expression in the nucleus.
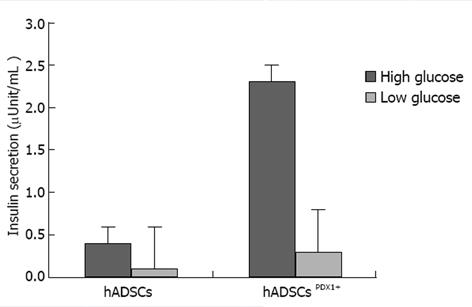
Figure 9 Insulin secretion assay in culture medium of human adipose tissue derived stem cellsPDX1+ and human adipose tissue derived stem cells after 3 wk.
It was performed using electrochemiluminescence test (Bahar lab, Tehran, Iran). Insulin concentration was increased to 2.32 fold in human adipose tissue derived stem cells (hADSCs)PDX1 compared to hADSCs control, when it was exposed to a high glucose concentration.
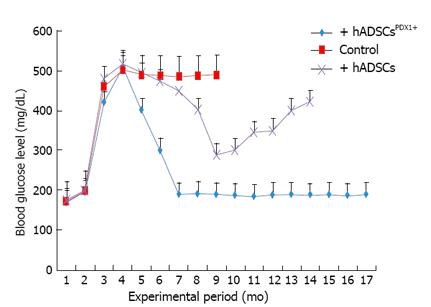
Figure 10 In vivo animal study of hyperglycemia.
Treatment by transplantation of human adipose tissue derived stem cellsPDX1+ and human adipose tissue derived stem cells (4 × 106 cells/rat). Before alloxan injection, the blood glucose level was 180 ± 20 and it reached 500 ± 20 mg/mL two weeks after injection. Six alloxan induced diabetic rats were transplanted with human adipose tissue derived stem cells (hADSCs)PDX1+ which resulted in decreasing the blood glucose level to 200 ± 20 mg/mL after 3 d. The blood glucose levels of 6 rats transplanted with hADSCs were decreased transiently to 300 ± 20 mg/mL after 10 d and increased again. Control group which consisted of 6 diabetic rats untransplanted were hyperglycemic and died within 6 mo.
-
Citation: Boroujeni ZN, Aleyasin A. Insulin producing cells established using non-integrated lentiviral vector harboring
PDX1 gene. World J Stem Cells 2013; 5(4): 217-228 - URL: https://www.wjgnet.com/1948-0210/full/v5/i4/217.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i4.217