Copyright
©2011 Baishideng Publishing Group Co.
World J Stem Cells. Feb 26, 2011; 3(2): 9-18
Published online Feb 26, 2011. doi: 10.4252/wjsc.v3.i2.9
Published online Feb 26, 2011. doi: 10.4252/wjsc.v3.i2.9
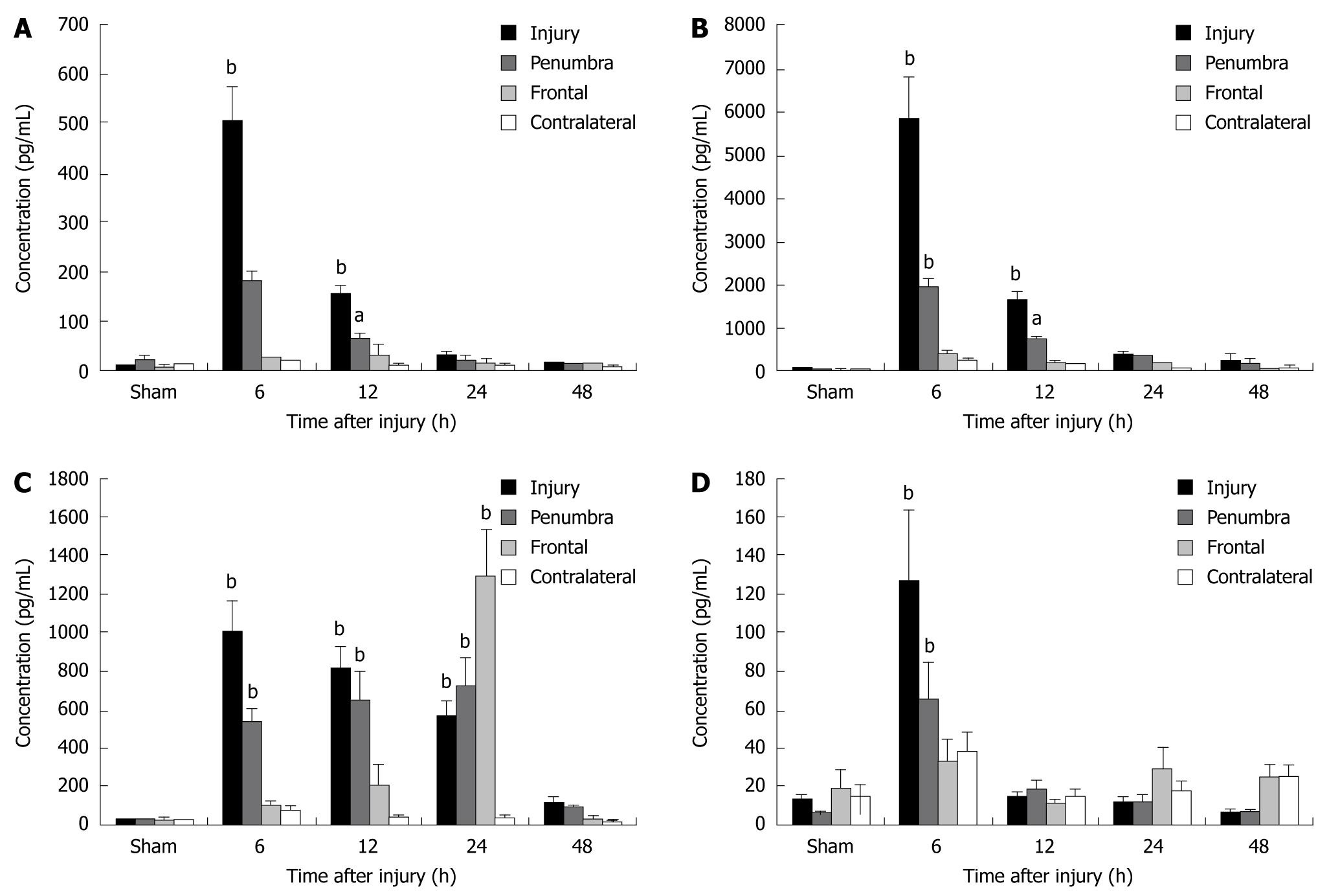
Figure 1 Elevated intracerebral cytokines identified in specific areas and at specific time points relative to the traumatic brain injury.
The proinflammatory cytokines interleukin (IL)-1α (A), IL-1β (B), IL-6 (C), and tumor necrosis factor-α (D) were significantly elevated 6 h after CCI in the injury and penumbral regions when compared with sham animals (bP < 0.01 for all). IL-1α, IL-1β, and IL-6 remained elevated through 12, 12 and 24 h, respectively (bP < 0.01 or aP < 0.05). In the frontal area, IL-6 was significantly increased at 24 h (33- to 50-fold; P < 0.01; Dunnett's test), but not at 6 or 12 h after traumatic brain injury. Reproduced with permission[22].
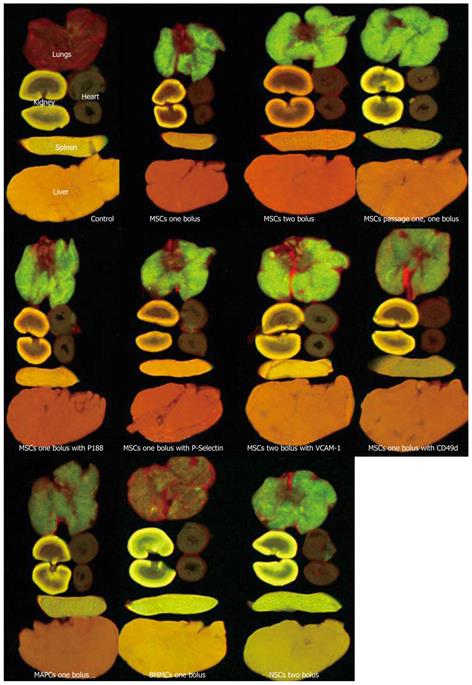
Figure 2 Fluorescent imaging of QDOT (green) labeled mesenchymal stromal cells, neuronal stem cells, multipotent adult progenitor cells, and bone marrow mononuclear cells after intravenous injection.
Less than 1% of mesenchymal stromal cells (MSCs) bypassed the lungs into the arterial circulation (as shown by high levels of green fluorescence). A two fold increase in pulmonary bypass was observed with neuronal stem cells (NSCs) and multipotent adult progenitor cells (MAPCs) with a 50 fold increase observed with bone marrow mononuclear cells (BMMCs). Reproduced with permission[48].
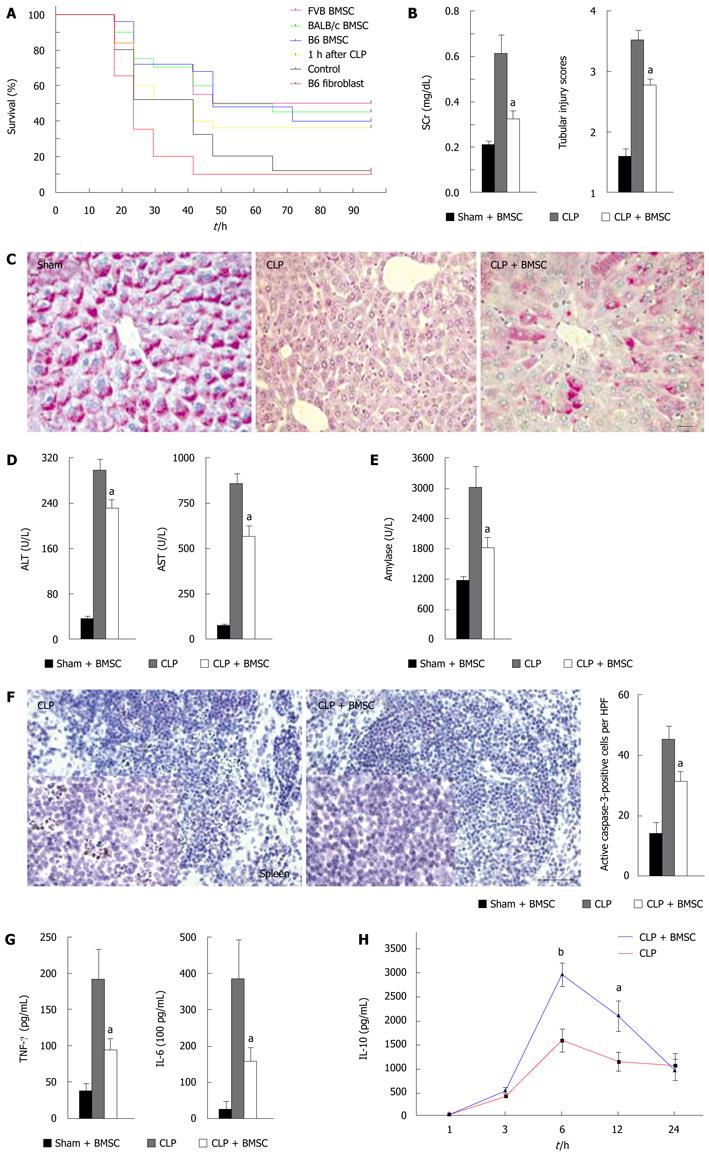
Figure 3 Effect of intravenous injection of BMSCs on the course of sepsis after cecal ligation and puncture.
A: Survival curves of mice after cecal ligation and puncture (CLP) and a variety of treatments using BMSCs from C57/BL6, FVB/NJ and BALB/c mice, as well as C57/BL6-derived fibroblasts; B: BMSC treatment effects on kidney function, as reflected by serum concentration of creatinine (SCr). The number of mice in all measurements is as follows: sham, n = 5; CLP, n = 13; CLP + BMSC, n = 14. Tubular injury scores are shown at right; C: Intense PAS staining of hepatocytes is shown after sham operation and BMSC treatment. No staining can be seen in CLP. After treatment (CLP + BMSC), the red staining by PAS in hepatocytes indicates partial glycogen storage capacity. Scale bar, 20 μm; D: Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations in the liver after sham and BMSC, CLP or CLP and BMSC treatment; E: Serum amylase concentrations after sham and BMSC, CLP or CLP and BMSC treatment; F: DAB staining of caspase-3 cells in untreated spleen sections and BMSC-treated spleen sections. A quantitative comparison between the numbers of apoptotic splenic cells in treated versus untreated mice (right) shows a significant decrease with BMSC treatment. Scale bar, 100 μm; G: Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 concentrations after sham and BMSC, CLP or CLP and BMSC treatment; H: Serum IL-10 concentrations at 3, 6 and 12 h after CLP. n = 8-11 at each time point. Error bars represent means ± SE; aP < 0.05; bP < 0.01. Reproduced with permission[36].
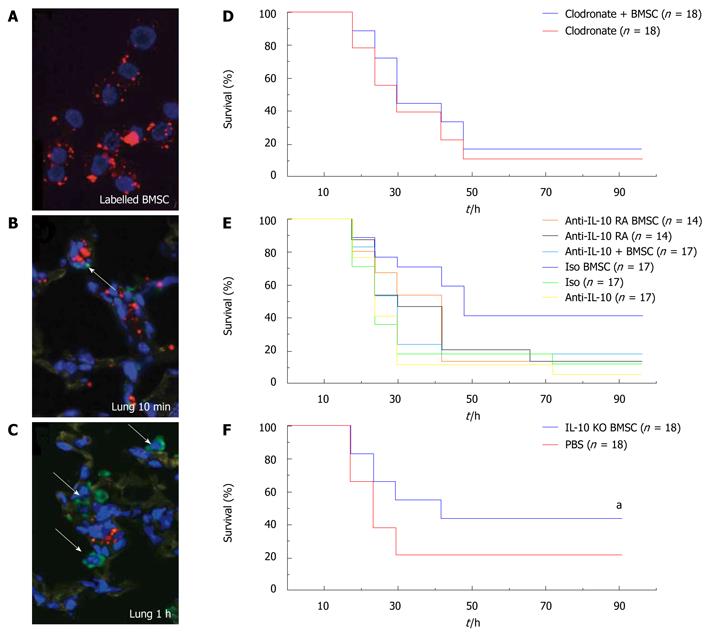
Figure 4 Fate of injected BMSCs and effect of BMSC treatment on survival of normal and immune cell-depleted mice.
A-C Immunohistochemical staining showing that BMSCs pre-labeled with Q-dot (red punctate staining; (A) travel to the lung (B) and take up residence in close proximity to macrophages (C); The latter cells were immunostained with an antibody to Iba1 (ionized calcium-binding adaptor molecule-1, a specific marker of the macrophage lineage47) and visualized with Alexa-Fluor-488 conjugated to a secondary antibody (green). Scale bar, 10 μm; (D-F) Summary of the effectiveness of BMSC treatment of mice genetically lacking or depleted of certain subsets of immune cells or soluble mediators. Survival curves show survival percentage of macrophage-depleted mice with or without BMSC treatment (D), survival percentage of BMSC-treated CLP mice and untreated mice after neutralizing IL-10 or blocking the IL-10 receptor (e) and survival percentage of after treatment with BMSCs derived from Il10-/- septic mice (F). aP < 0.05. Reproduced with permission[36].
-
Citation: Walker PA, Letourneau PA, Bedi S, Shah SK, Jimenez F, Jr CSC. Progenitor cells as remote "bioreactors": Neuroprotection
via modulation of the systemic inflammatory response. World J Stem Cells 2011; 3(2): 9-18 - URL: https://www.wjgnet.com/1948-0210/full/v3/i2/9.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v3.i2.9