Copyright
©The Author(s) 2025.
World J Stem Cells. Jul 26, 2025; 17(7): 101929
Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.101929
Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.101929
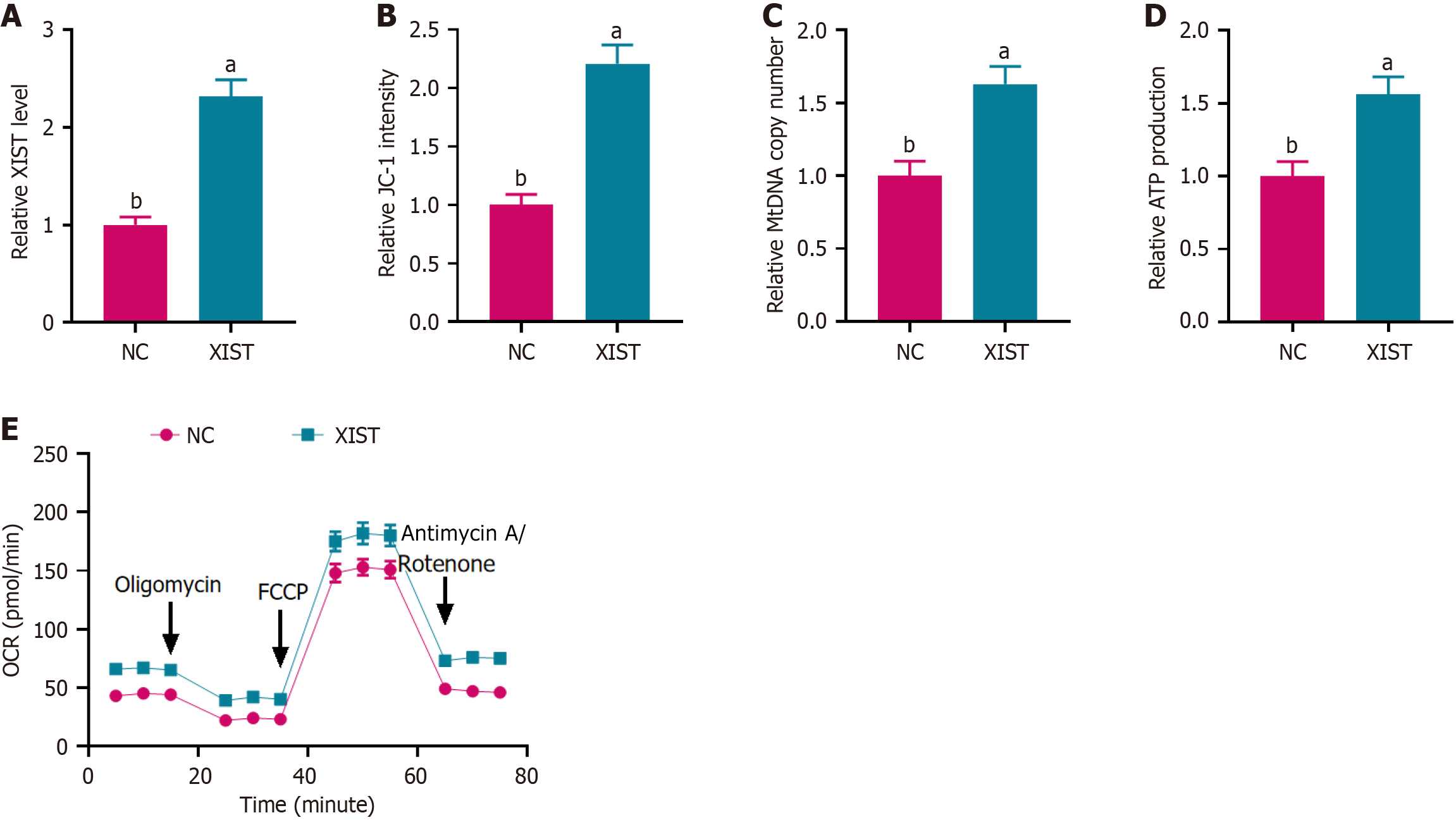
Figure 1 X inactive-specific transcript overexpression enhanced mitochondrial function in neural stem cells.
A: Relative X inactive-specific transcript (XIST) expression level measured by PCR, indicating successful overexpression of XIST in neural stem cells; B: JC-1 staining assay showing the relative JC-1 intensity, reflecting mitochondrial membrane potential; C: Relative mitochondrial DNA copy number assessed by PCR, indicating an increase in mitochondrial biogenesis; D: Relative ATP production levels measured using an ATP assay kit, demonstrating enhanced ATP generation; E: Oxygen consumption rate result showed increased mitochondrial respiration in XIST-overexpressing neural stem cells. Data are presented as mean ± SD. aP < 0.01, bP < 0.001. XIST: X inactive-specific transcript; NC: Negative control; mtDNA: Mitochondrial DNA; OCR: Oxygen consumption rate; FCCP: Carbonyl cyanide 4-(trifluorome
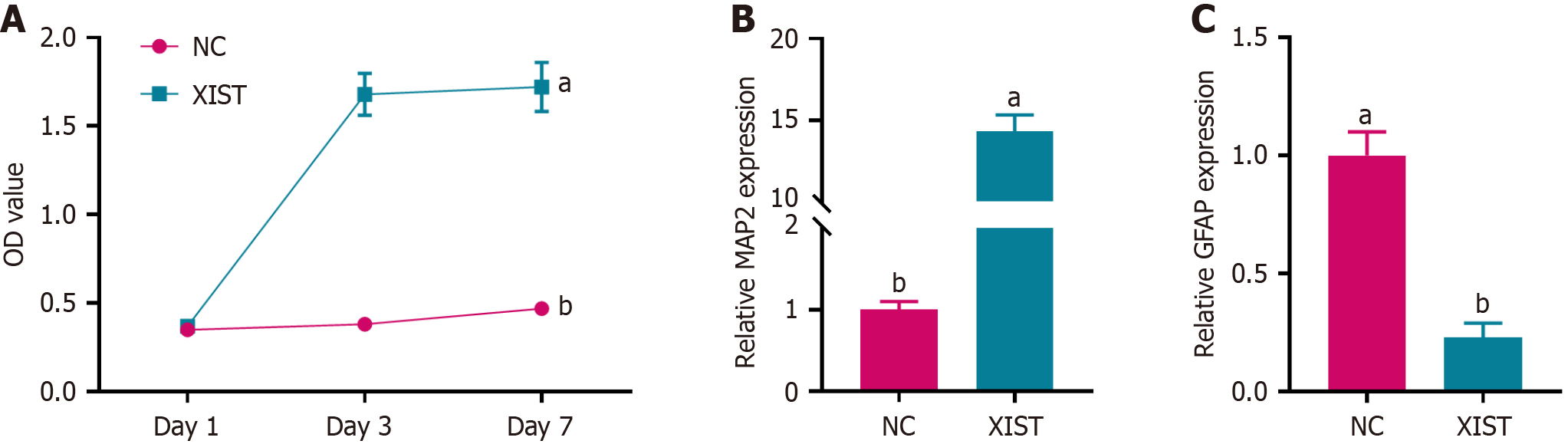
Figure 2 X inactive-specific transcript enhanced neural stem cell proliferation and influenced cell differentiation.
A: CCK-8 assay showed the proliferation of neural stem cells transfected with X inactive-specific transcript (XIST) compared with the negative control (NC) at days 1, 3, and 7; B: Relative expression of the neuronal marker microtubule-associated protein 2 at day 7, showing upregulation in the XIST group compared to NC; C: Relative expression of the astrocytic marker glial fibrillary acidic protein at day 7, showing downregulation in the XIST group compared to NC. Data are presented as mean ± SD. aP < 0.01, bP < 0.001. XIST: X inactive-specific transcript; NC: Negative control; MAP2: Microtubule-associated protein 2; GFAP: Glial fibrillary acidic protein.
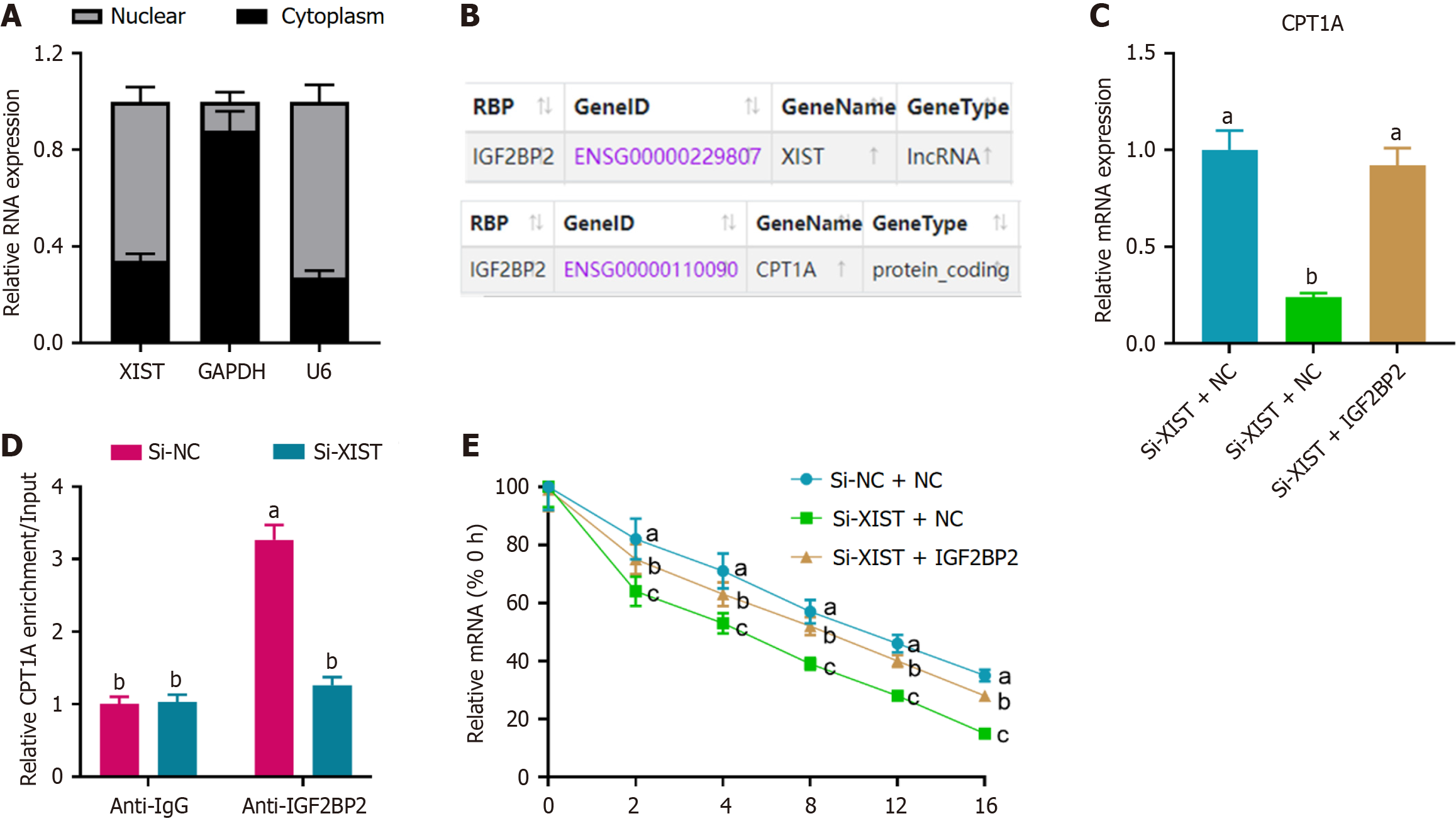
Figure 3 X inactive-specific transcript modulated carnitine palmitoyl transferase 1A expression through interaction with insulin-like growth factor 2 mRNA binding protein 2.
A: Cytoplasmic and nuclear RNA fractionation showing that X inactive-specific transcript (XIST) is primarily localized in the nucleus of neural stem cells. GAPDH and U6 served as cytoplasmic and nuclear controls, respectively; B: Bioinformatics prediction from StarBase indicating potential regulation of carnitine palmitoyl transferase 1A (CPT1A) by XIST through the insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) pathway; C: Relative mRNA expression of CPT1A upon XIST knockdown and subsequent rescue by IGF2BP2 knockdown, as determined by real-time quantitative PCR. aP < 0.05, si-XIST + negative control vs si-XIST + IGF2BP2; bP > 0.05; D: RNA immunoprecipitation assay showing CPT1A enrichment on IGF2BP2, which was reversed by XIST knockdown. aP < 0.05, si-negative control vs si-XIST; bP > 0.05; E: Analysis of CPT1A mRNA stability, indicating that XIST knockdown reduced CPT1A stability, an effect reversed by IGF2BP2 knockdown. aP < 0.05, si-negative control + negative control vs si-XIST + negative control; bP > 0.05; cP < 0.05, si-XIST + negative control vs si-XIST + IGF2BP2. Data are presented as mean ± SD. XIST: X inactive-specific transcript; NC: Negative control; CPT1A: Carnitine palmitoyl transferase 1A; IGF2BP2: Insulin-like growth factor 2 mRNA binding protein 2.
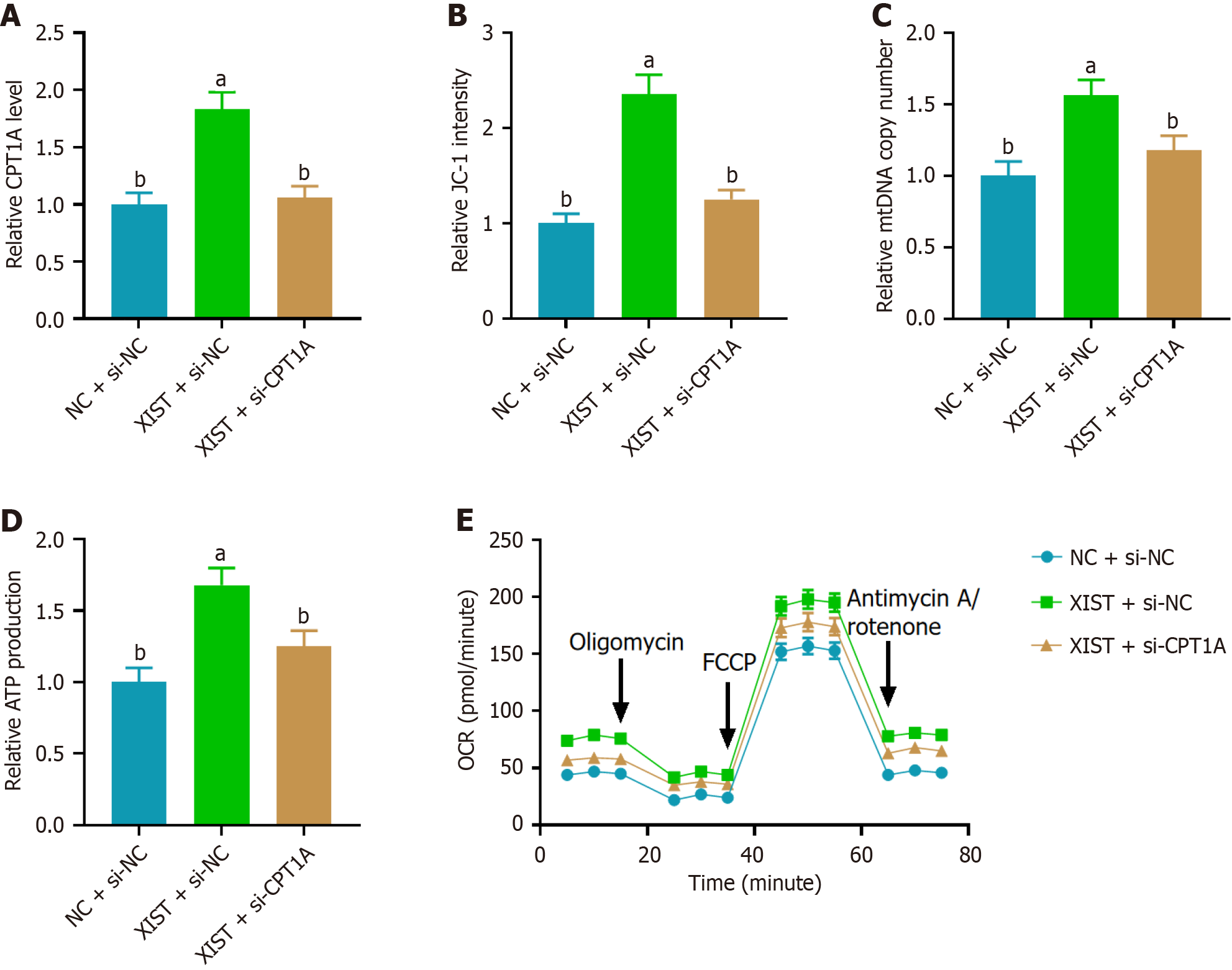
Figure 4 Carnitine palmitoyl transferase 1A knockdown reversed the effects of X inactive-specific transcript overexpression on mitochondrial oxidative phosphorylation in neural stem cells.
A: Relative carnitine palmitoyl transferase 1A (CPT1A) mRNA levels determined by real-time quantitative PCR in neural stem cells with X inactive-specific transcript (XIST) overexpression, showing upregulation of CPT1A and reversal by si-CPT1A; B: Mitochondrial membrane potential assessed by the JC-1 assay, indicating increased mitochondrial membrane potential with XIST overexpression and reversal by si-CPT1A; C: Relative mitochondrial DNA copy number measured by PCR, showing an increase with XIST overexpression and reversal by si-CPT1A; D: Relative ATP production in neural stem cells, demonstrating that XIST overexpression enhances ATP production, with reversal by si-CPT1A; E: Oxygen consumption rate result indicated that XIST overexpression enhanced mitochondrial oxidative phosphorylation, with reversal by si-CPT1A. Data are presented as mean ± SD. aP < 0.01, bP < 0.001. XIST: X inactive-specific transcript; NC: Negative control; CPT1A: Carnitine palmitoyl transferase 1A; mtDNA: Mitochondrial DNA; OCR: Oxygen consumption rate; FCCP: Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone.
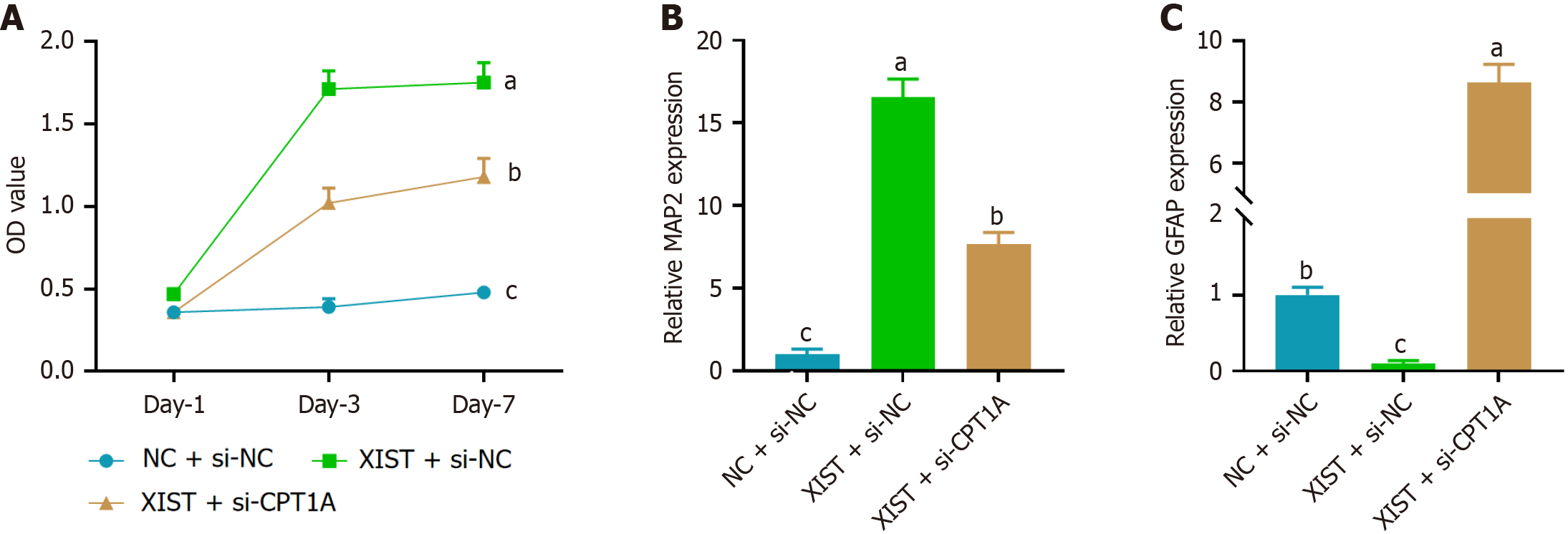
Figure 5 Carnitine palmitoyl transferase 1A knockdown reversed the effects of X inactive-specific transcript overexpression on neural differentiation in neural stem cells.
A: CCK-8 assay showed that X inactive-specific transcript (XIST) overexpression promoted neural stem cell proliferation, while carnitine palmitoyl transferase 1A (CPT1A) knockdown (si-CPT1A) reversed this effect; B: Relative expression of the neuronal marker microtubule-associated protein 2 at day 7, showing that XIST overexpression significantly upregulated microtubule-associated protein 2 expression, an effect reversed by CPT1A knockdown; C: Relative expression of the astrocytic marker glial fibrillary acidic protein at day 7, showing that XIST overexpression downregulated glial fibrillary acidic protein expression, with CPT1A knockdown reversing this effect. Data are presented as mean ± SD. aP < 0.05, bP < 0.01, cP < 0.001. XIST: X inactive-specific transcript; NC: Negative control; CPT1A: Carnitine palmitoyl transferase 1A; MAP2: Microtubule-associated protein 2; GFAP: Glial fibrillary acidic protein.
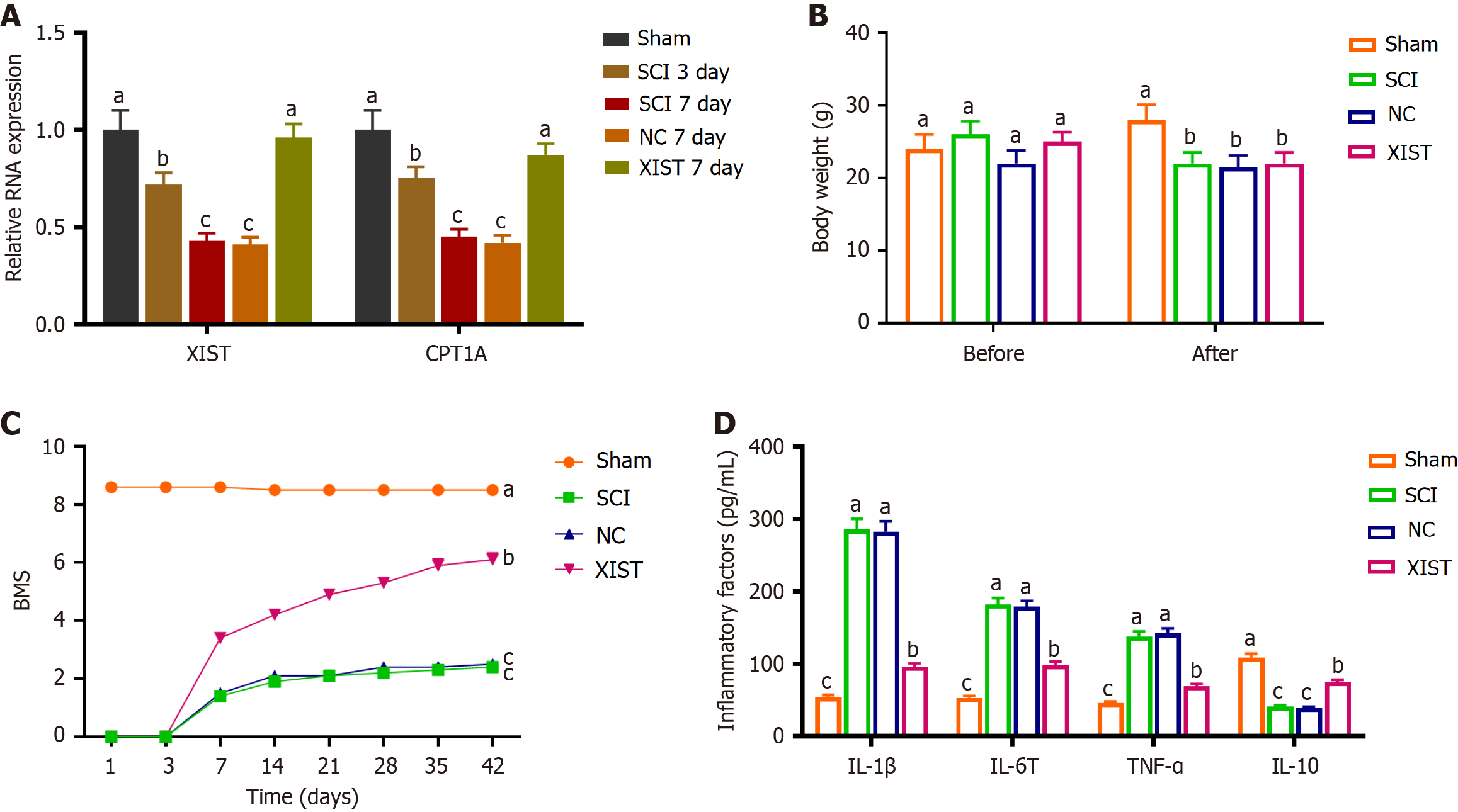
Figure 6 X inactive-specific transcript alleviated spinal cord injury by modulating neural stem cell differentiation via the insulin-like growth factor 2 mRNA binding protein 2/carnitine palmitoyl transferase 1A pathway.
A: Relative RNA expression of X inactive-specific transcript (XIST) and carnitine palmitoyl transferase 1A on days 3 and 7 post-spinal cord injury (SCI), showing reduced expression in the SCI groups and elevated expression in the XIST day 7 group compared with the negative control (NC) day 7 group; B: Body weight measurements before surgery and 6 weeks after surgery, showing no significant differences in weight among the SCI, NC, and XIST groups; C: Basso Mouse Scale scores demonstrated a significant decline in the SCI group and a notable improvement in the XIST group compared with the NC group; D: ELISA measurement of inflammatory cytokine levels in spinal cord tissues, showing increased inflammation in the SCI model and reversal by XIST treatment. Data are presented as mean ± SD. aP < 0.05 indicates sham vs spinal cord injury (day 3 or 7), bP < 0.05 indicates spinal cord injury vs X inactive-specific transcript (day 7), cP < 0.05 indicates X inactive-specific transcript vs negative control (day 7). XIST: X inactive-specific transcript; NC: Negative control; CPT1A: Carnitine palmitoyl transferase 1A; SCI: Spinal cord injury; BMS: Basso Mouse Scale; IL: Interleukin; TNF: Tumor necrosis factor.
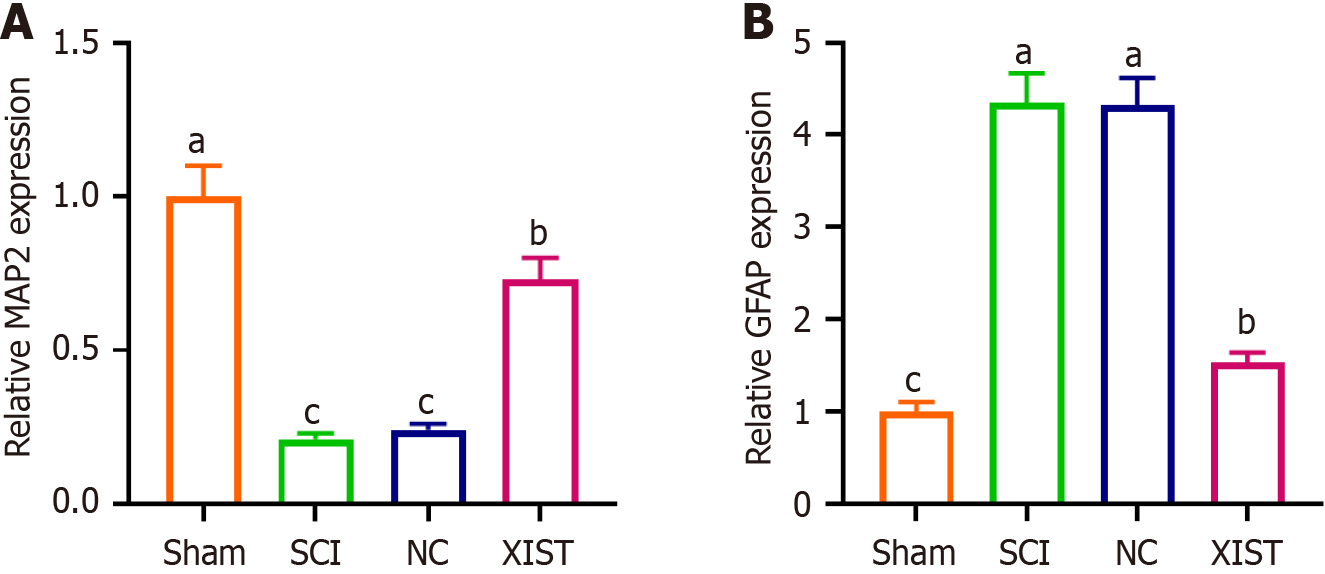
Figure 7 X inactive-specific transcript reverses the effects of spinal cord injury on microtubule-associated protein 2 and glial fibrillary acidic protein expression.
A: Real-time quantitative PCR analysis of microtubule-associated protein 2 expression, showing a significant reduction in the spinal cord injury group and reversal by X inactive-specific transcript treatment; B: Real-time quantitative PCR analysis of glial fibrillary acidic protein expression, showing a significant increase in the spinal cord injury group and reversal by X inactive-specific transcript treatment. Data are presented as mean ± SD. aP < 0.05 indicates sham vs spinal cord injury, bP < 0.05 indicates spinal cord injury vs X inactive-specific transcript, cP < 0.05 indicates negative control vs X inactive-specific transcript. XIST: X inactive-specific transcript; NC: Negative control; SCI: Spinal cord injury; MAP2: Microtubule-associated protein 2; GFAP: Glial fibrillary acidic protein.
- Citation: Zeng SX, Ye JT, Huang SH, Liu RX. X inactive-specific transcript regulates mitochondrial function and neuronal differentiation of stem cells via IGF2BP2/CPT1A axis in models of spinal cord injury. World J Stem Cells 2025; 17(7): 101929
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/101929.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.101929