Copyright
©The Author(s) 2025.
World J Stem Cells. Mar 26, 2025; 17(3): 99472
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.99472
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.99472
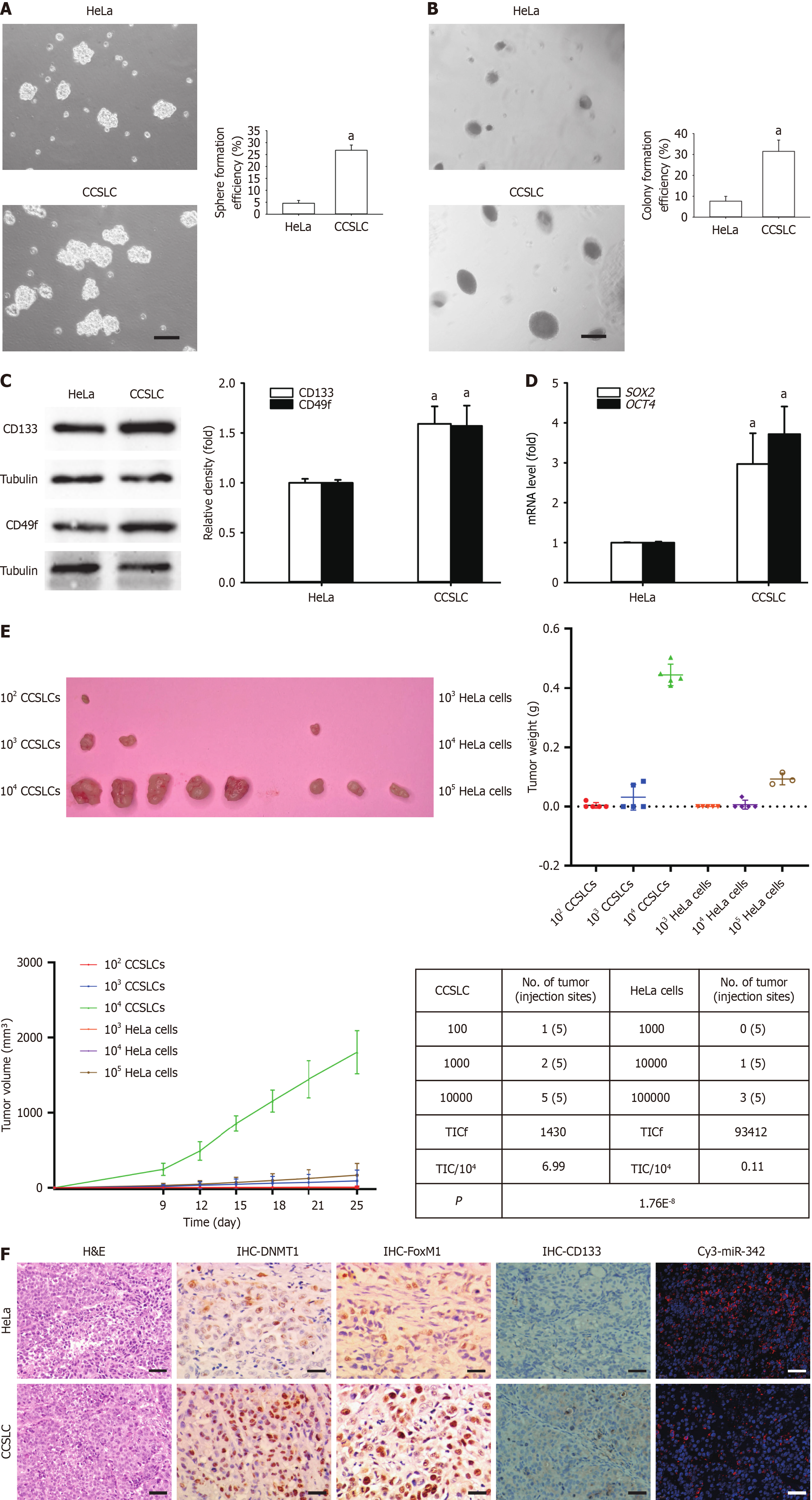
Figure 1 Comparing the self-renewal related stemness between HeLa cells and corresponding cervical cancer stem cell-like cells.
A and B: Representative images of spheres (A) and colonies (B) (left) (scale bars = 100 μm); sphere formation efficiency and colony formation efficiency (right) in HeLa cells and their cervical cancer stem cell-like cells (CCSLCs); C: Expression levels of CD133 and CD49f in HeLa cells and their CCSLCs; D: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA levels in HeLa cells and their CCSLCs; E: In vivo carcinogenicity between HeLa cells and their CCSLCs in the nude mice xenograft model, including the weight of xenograft tumors harvested from nude mice (upper right), growth curves of tumor xenografts (bottom left), and tumor initiated cell frequency (bottom right); F: Histology, expression of DNA methyltransferase 1 (DNMT1), forkhead box M1 (FOXM1), and CD133 proteins and microRNA (miR)-342-3p (scale bars = 50 μm). Data were obtained from xenografts at 5 inoculation sites (n = 5) per group. aP < 0.05 vs HeLa cells (n = 3). TICf: Tumor initiated cell frequency; H&E: Hematoxylin and eosin; IHC: Immunohistochemistry.
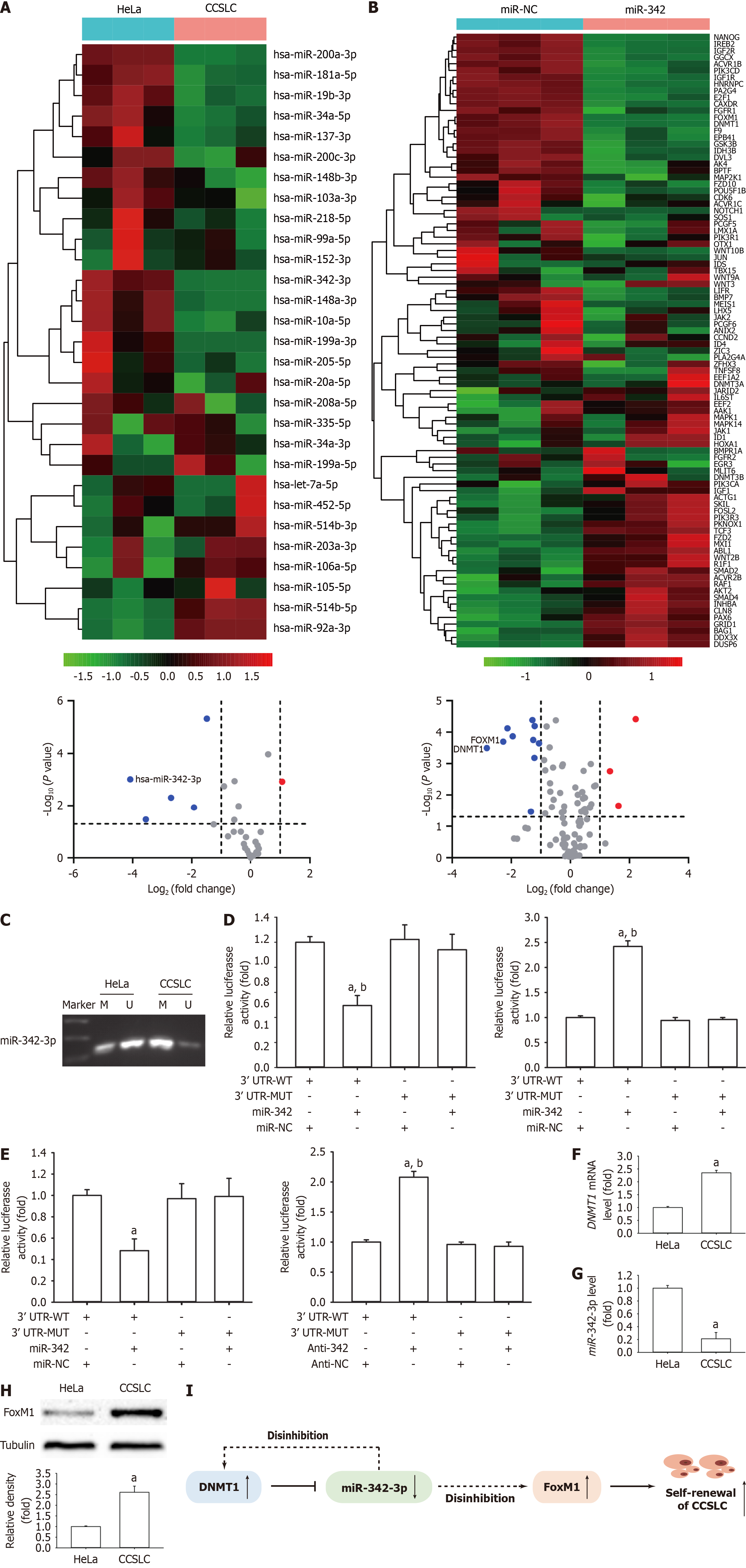
Figure 2 DNA methyltransferase 1 and forkhead box M1 as the direct targets of miR-342-3p in HeLa-derived cervical cancer stem cell-like cells.
A: Heatmaps depict the relative expression levels of all 29 tumor microRNAs (miRNAs) in HeLa cells and their responding cervical cancer stem cell-like cells (CCSLCs). The color scale represents the expression levels of miRNA as upregulation (red), downregulation (green), or middle expression (light color) across all samples. Lower volcano plot analysis showed differentially expressed (P < 0.005, student’s t-test, log2 fold change > 2) miRNA in HeLa cells and their responding CCSLCs; B: Visual representation displaying the expression levels of all 92 genes investigated in this study through heatmap analysis. Scale: Relative expression levels were normalized for internal control. Lower volcano plot showing the differentially expressed mRNAs (P < 0.005, student’s t-test, log2 fold change > 2) in HeLa-derived CCSLCs treated with miRNA (miR)-negative control or miR-342 (n = 3); C: MiR-342-3p promoter methylation in HeLa cells and their CCSLCs; D: The relative luciferase activity in HeLa-derived CCSLCs was determined after the DNA methyltransferase 1 (DNMT1) 3’-untranslated regions (3’-UTRs) or mutant (MUT) plasmids were cotransfected with miR-342-3p mimics or inhibitors or negative control; E: The relative luciferase activity in HeLa-derived CCSLCs was determined after the forkhead box M1 (FOXM1) 3’ UTR or mutant plasmids were cotransfected with miR-342-3p mimics or inhibitors or the negative control; F: DNMT1 mRNA level in HeLa cells and its corresponding CCSLCs; G: MiR-342-3p level in HeLa cells and its corresponding CCSLCs; H: FoxM1 protein amounts in HeLa cells and its corresponding CCSLCs, with α-tubulin as a loading control; I: Schematic representation of the pathomechanism by which DNMT1/miR-342-3p/FOXM1 axis promotes self-renewal-related stemness in CCSLCs. aP < 0.05 vs HeLa-derived cervical cancer stem cell-like cells cotransfected with DNMT1 3’-UTR with miR-negative; bP < 0.05 vs HeLa-derived cervical cancer stem cell-like cells cotransfected of mutant plasmid in the presence or absence of miR-342 mimics (n = 3). WT: Wildtype; M: Methylated; U: Unmethylated.
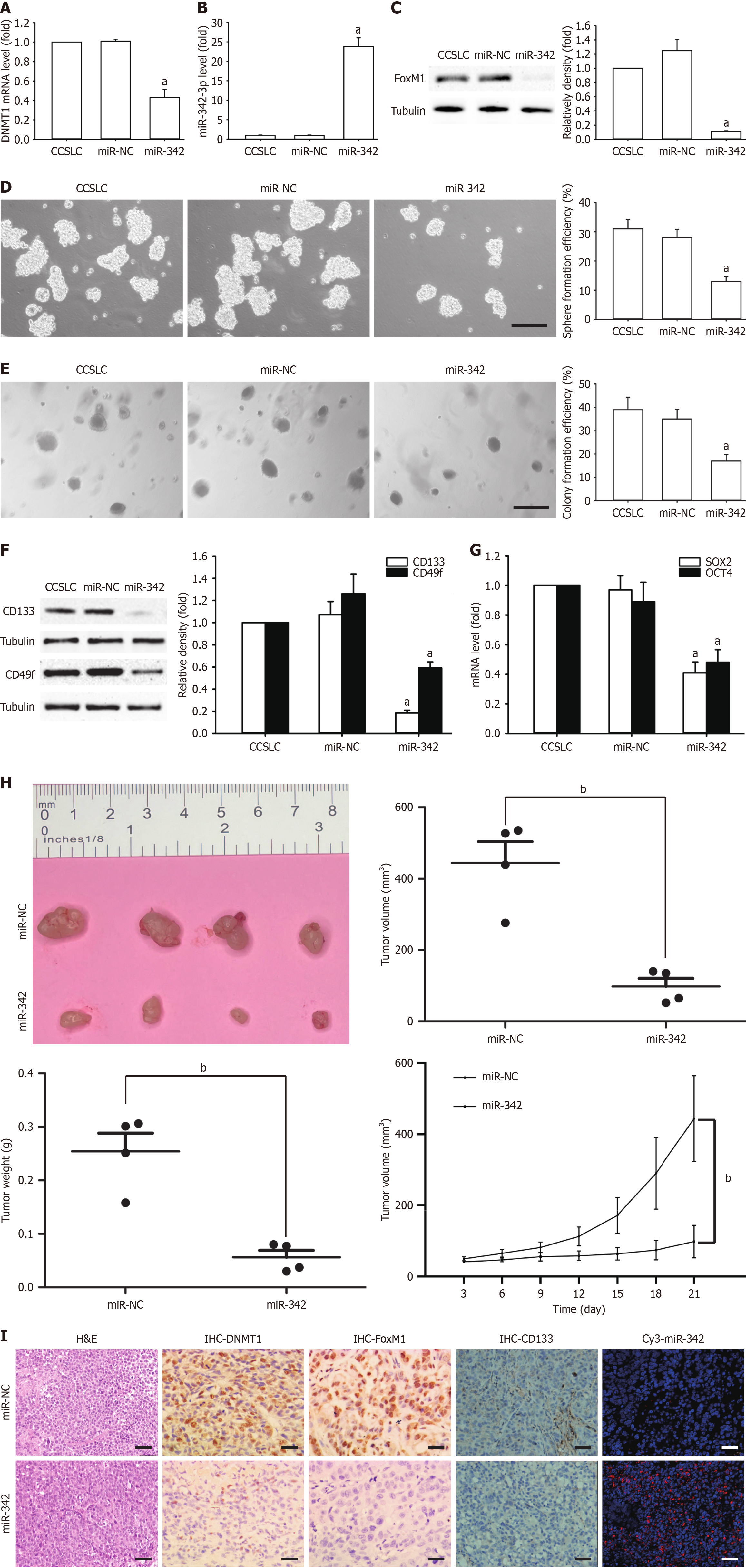
Figure 3 Effect of miR-342-3p mimic on self-renewal-related stemness of HeLa-derived cervical cancer stem cell-like cells.
A-C: DNA methyltransferase 1 (DNMT1) mRNA level (A), microRNA (miR)-342-3p level (B), forkhead box M1 (FoxM1) protein amounts in cervical cancer stem cell-like cells (CCSLCs) transfected with miR-342-3p mimic (C); D and E: Representative images of spheres and colonies (left) (scale bars = 100 μm); sphere formation efficiency and colony formation efficiency (right) in CCSLCs transfected with miR-342-3p mimic; F: CD133 and CD49f protein amount; G: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA amounts in CCSLCs transfected with miR-342-3p mimic; H: Subcutaneous xenografts of HeLa-derived CCSLCs (1 × 105) treated with miR-negative control (miR-NC) or miR-342-3p mimic (miR-342) (left); the comparison of tumor volume (middle left), tumor weight (middle right), and growth curves of tumor xenografts (right) between CCSLCs treated with miR-NC or miR-342; I: Micrographs of hematoxylin and eosin staining and immunohistochemistry to detect the expression of DNMT1, FoxM1, and CD133 proteins as well as in situ immunofluorescent hybridization for miR-342-3p (scale bars = 50 μm). Data were obtained from xenografts with four mice per group (n = 4). aP < 0.05 vs cervical cancer stem cell-like cells transfected with miR-NC (n = 4); bP < 0.01 vs cervical cancer stem cell-like cells treated with miR-NC. H&E: Hematoxylin and eosin; IHC: Immunohistochemistry.
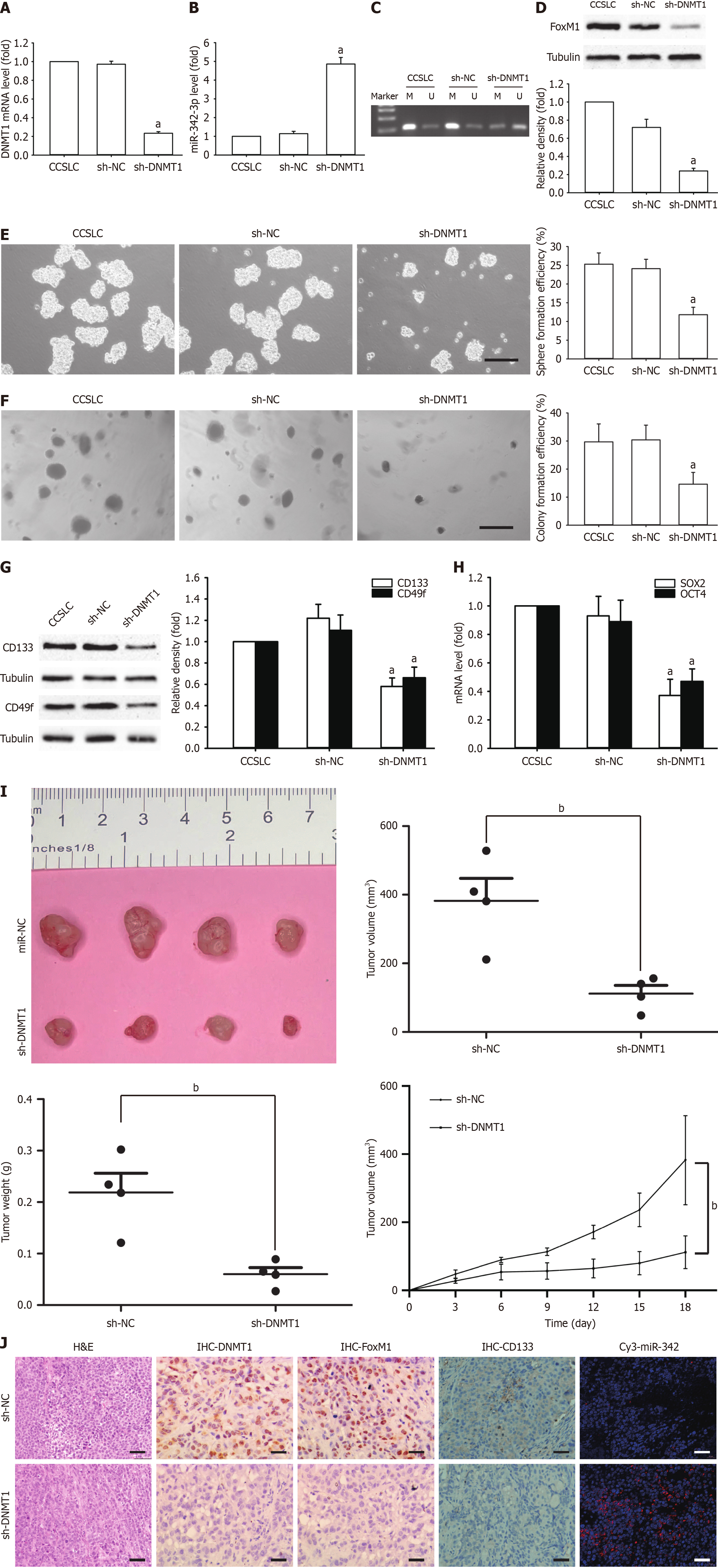
Figure 4 Effect of DNA methyltransferase 1 shRNA on cervical cancer stem cell-like cell characteristics in HeLa-derived cervical cancer stem cell-like cells.
A: DNA methyltransferase 1 (DNMT1) mRNA level in cervical cancer stem cell-like cells (CCSLCs) transfected with DNMT1 shRNA; B: MicroRNA (miR)-342-3p levels in CCSLCs transfected with DNMT1 shRNA; C: MiR-342-3p promoter methylation in CCSLCs transfected with DNMT1 shRNA; D: Forkhead box M1 (FoxM1) protein amounts in CCSLCs transfected with DNMT1 shRNA, with α-tubulin as a loading control; E and F: Representative images of spheres (E) and colonies (F) (left) (scale bars = 100 μm). Sphere formation efficiency and colony formation efficiency in CCSLCs transfected with DNMT1 shRNA (right); G: CD133 and CD49f protein amount in CCSLCs transfected with DNMT1 shRNA, with α-tubulin as a loading control; H: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA amounts in CCSLCs transfected with DNMT1 shRNA; I: Subcutaneous xenografts of CCSLCs (1 × 105) treated with sh-negative control (sh-NC) or sh-DNMT1 (left); the comparison of tumor volume (middle left), tumor weight (middle right), and growth curves of tumor xenografts (right) between CCSLCs treated with sh-NC and sh-DNMT1; J: Micrographs of hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) to detect the expression of DNMT1, FoxM1, and CD133 proteins as well as in situ immunofluorescence hybridization for miR-342-3p (scale bars = 50 μm). Data were obtained from xenografts with four mice per group. aP < 0.05 vs CCSLCs transfected with sh-NC (n = 4); bP < 0.001 vs CCSLCs treated with sh-NC. M: Methylated; U: Unmethylated.
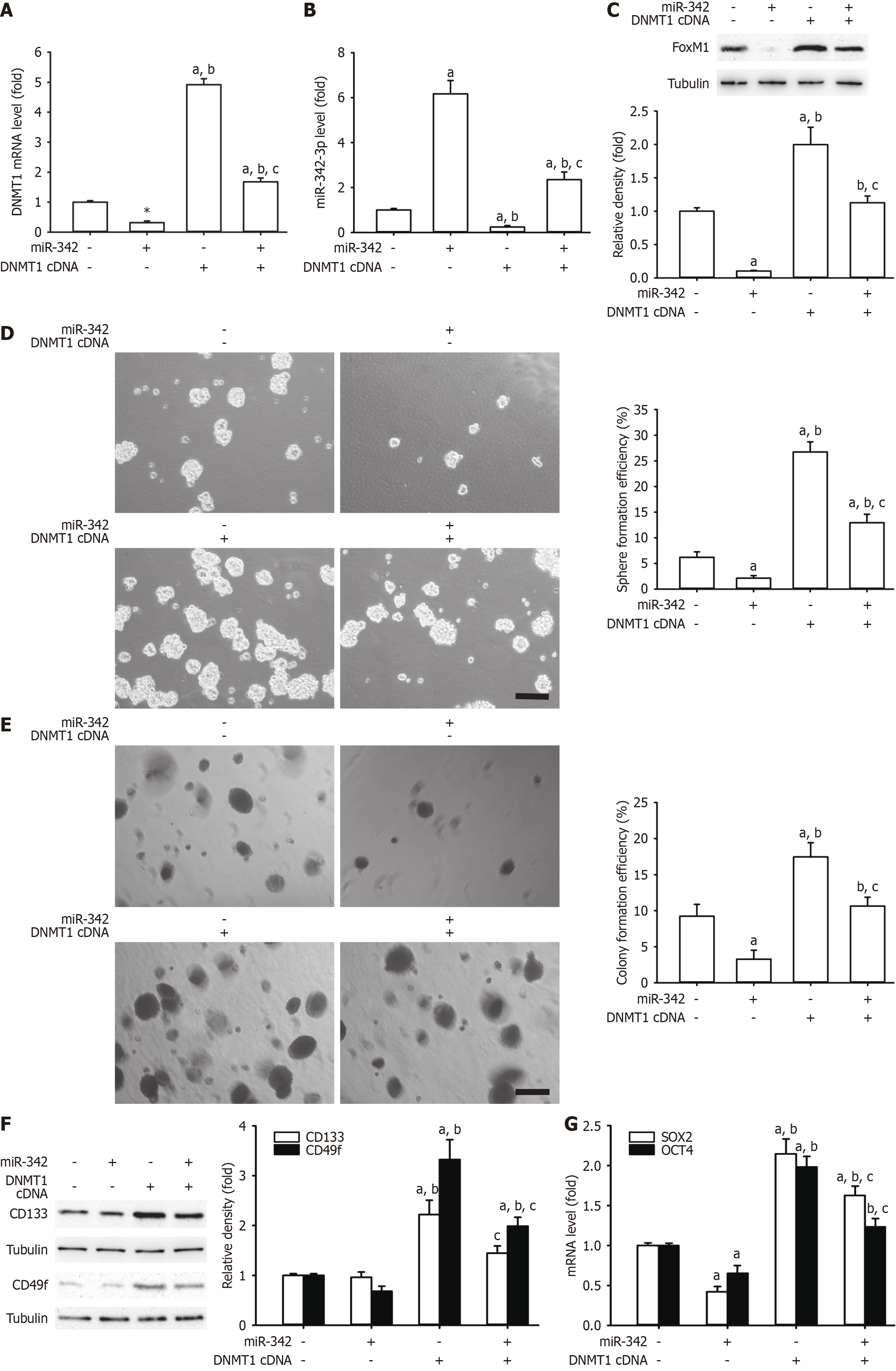
Figure 5 Overexpression of DNA methyltransferase 1 reversed the inhibitory effects of microRNA-342 on cervical cancer stem cell-like cell self-renewal in HeLa cells.
A and B: Restoration of DNA methyltransferase 1 (DNMT1) could reverse its mRNA level inhibition (A) and upregulation of microRNA (miR)-342-3p expression (B); C-E: Downregulation of forkhead box M1 (FoxM1) protein expression with α-tubulin as a loading control led to attenuation of cervical cancer stem cell-like cell self-renewal characteristics, including sphere formation efficiency and colony formation efficiency (D and E); F and G: CD133 and CD49f protein expressions with α-tubulin as a loading control and SRY-box transcription factor 2 (SOX2) (G) and octamer-binding transcription factor 4 (OCT4) mRNA expression caused by miR-342-3p mimics in HeLa cells. aP < 0.05 vs HeLa cells; bP < 0.05 vs HeLa cells transfected with miR-342-3p mimic alone; cP < 0.05 vs HeLa cells transfected with DNA methyltransferase 1 cDNA alone (n = 3).
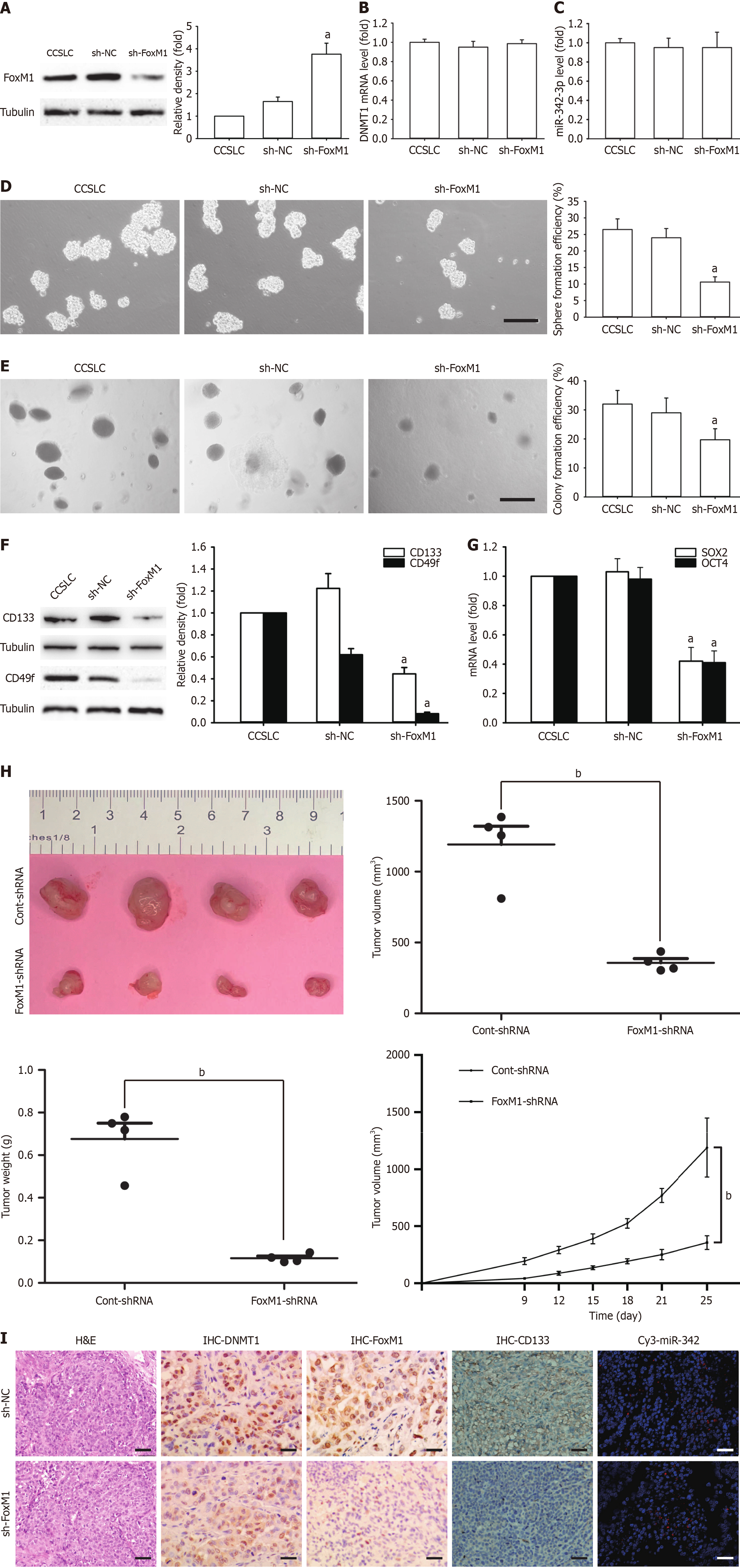
Figure 6 Effects of forkhead box M1 shRNA on cervical cancer stem cell-like cell self-renewal of HeLa derived cervical cancer stem cell-like cells.
A: Forkhead box M1 (FoxM1) protein in cervical cancer stem cell-like cells (CCSLCs) transfected with FoxM1 shRNA, with α-tubulin as a loading control; B: DNA methyltransferase 1 (DNMT1) mRNA level in CCSLCs transfected with FoxM1 shRNA; C: MicroRNA (miR)-342-3p levels in CCSLCs transfected with FoxM1 shRNA; D and E: Representative images of spheres and colonies (left) (scale bars = 100 μm); sphere formation efficiency and colony formation efficiency (right) in CCSLCs transfected with FoxM1 shRNA; F: CD133 and CD49f protein amount in CCSLCs transfected with DNMT1 shRNA with α-tubulin as a loading control; G: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA amounts in CCSLCs transfected with FOXM1 shRNA; H: Subcutaneous xenografts of HeLa-derived CCSLCs (1 × 105) treated with sh-negative control (sh-NC) or sh-FOXM1 (left); comparison of tumor volume (middle left), tumor weight (middle right), and growth curves of tumor xenografts (right) between CCSLCs treated with sh-NC or sh-FOXM1; I: Micrographs of hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) to detect the expression of DNMT1, FoxM1, and CD133 proteins as well as in situ immunofluorescent hybridization for miR-342-3p (scale bars = 50 μm). Data were obtained from xenografts with four mice per group (n = 4). aP < 0.05 vs CCSLCs transfected with sh-NC; bP < 0.001 vs CCSLCs treated with sh-NC. Cont: Control.
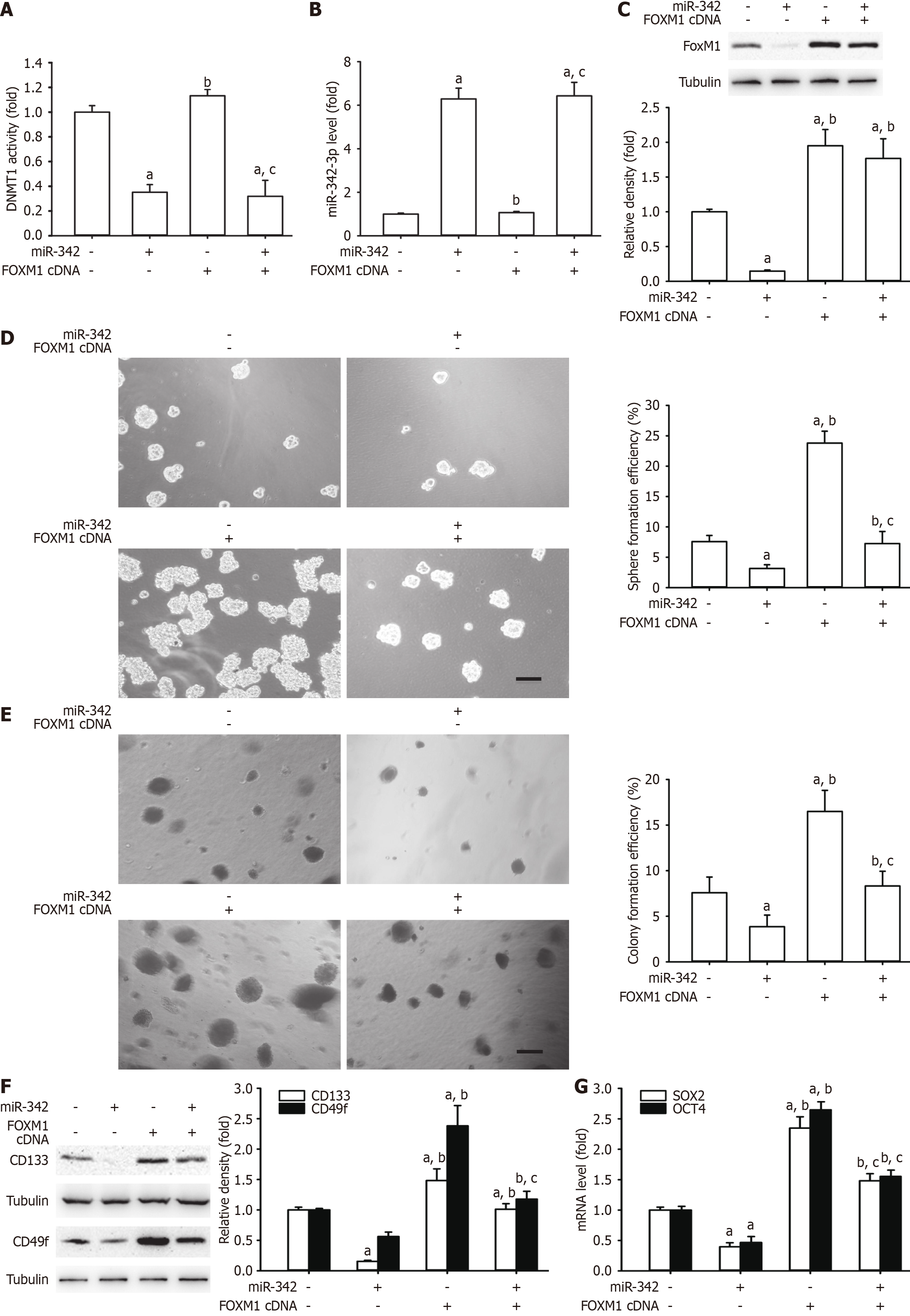
Figure 7 Overexpression of forkhead box M1 reversed the inhibitory effect of microRNA-342 on cervical cancer stem cell-like cell self-renewal in HeLa cells.
A-C: Restoration of forkhead box M1 (FOXM1) did not alter DNA methyltransferase 1 (DNMT1) (A) and microRNA (miR)-342-3p expression (B) but could reverse FOXM1 protein downregulation with α-tubulin as a loading control (C); D and E: Attenuation of cervical cancer stem cell-like cell characteristics, including sphere formation efficiency and colony formation efficiency; F: CD133 and CD49f protein expression with α-tubulin as a loading control; G: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA levels caused by miR-342-3p mimics in HeLa cells. aP < 0.05 vs HeLa cells; bP < 0.05 vs HeLa cells transfected with miR-342-3p mimic alone; cP < 0.05 vs HeLa cells transfected with FOXM1 cDNA alone (n = 3).
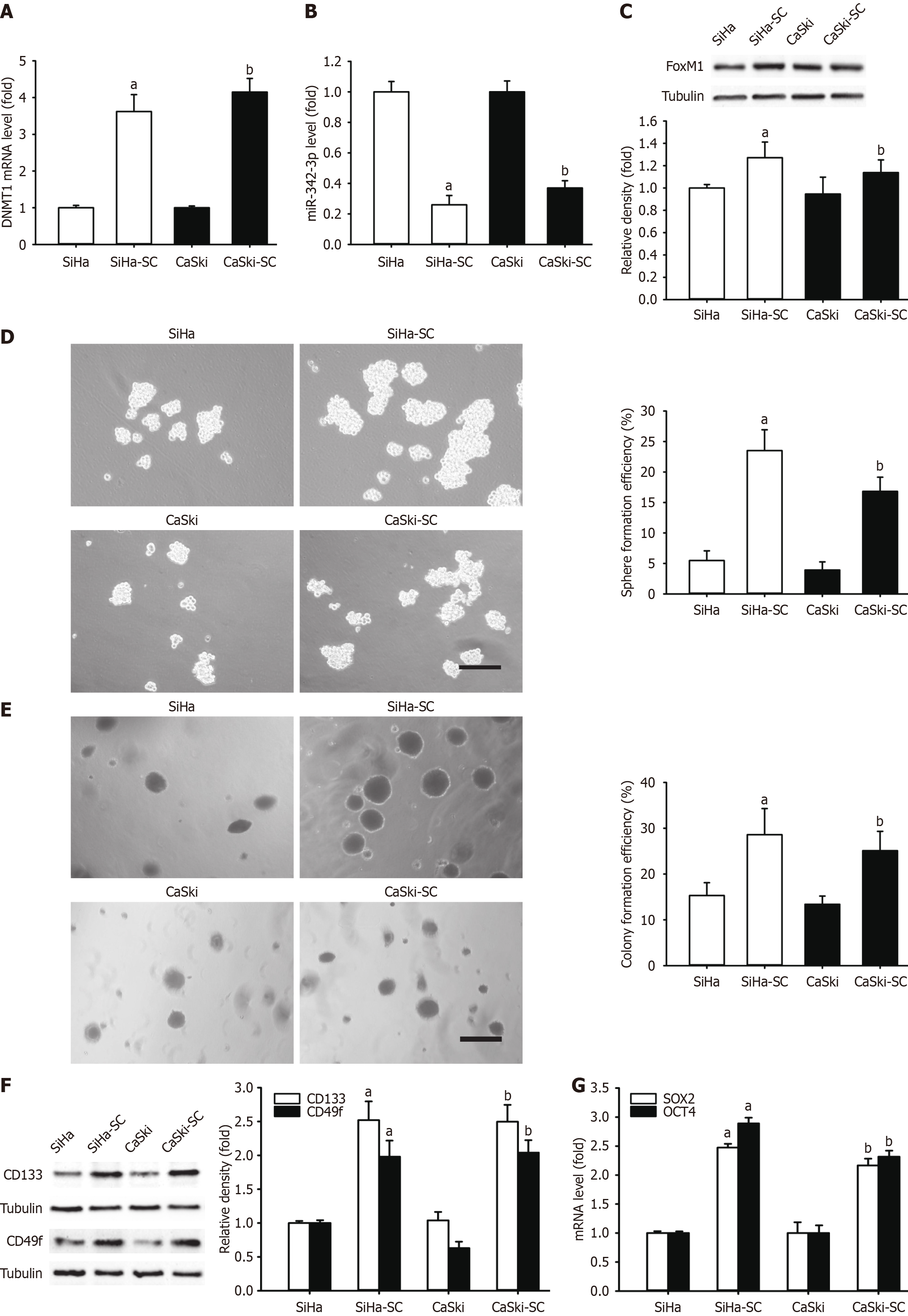
Figure 8 Comparing self-renewal related stemness between SiHa or CaSki cells and the corresponding cervical cancer stem cell-like cells.
A: DNA methyltransferase 1 (DNMT1) mRNA level in SiHa and CaSki cells and their corresponding cervical cancer stem cell-like cells (CCSLCs); B: MicroRNA (miR)-342-3p level in SiHa and CaSki cells and their corresponding CCSLCs; C: Forkhead box M1 (FOXM1) protein expression level SiHa and CaSki cells and their corresponding CCSLCs with α-tubulin as a loading control; D and E: Representative images of spheres and colonies (left) (scale bars = 100 μm); sphere formation efficiency and colony formation efficiency (right) in SiHa and CaSki cells and their corresponding CCSLCs; F: CD133 and CD49f protein amount with α-tubulin as a loading control; G: SRY-box transcription factor 2 (SOX2) and octamer-binding transcription factor 4 (OCT4) mRNA levels in SiHa and CaSki cells and their corresponding CCSLCs. aP < 0.05 vs SiHa cells; bP < 0.05 vs CaSki cells (n = 3). SC: Stem cell.
- Citation: Cao XZ, Zhang YF, Song YW, Yuan L, Tang HL, Li JY, Qiu YB, Lin JZ, Ning YX, Wang XY, Xu Y, Lin SQ. DNA methyltransferase 1/miR-342-3p/Forkhead box M1 signaling axis promotes self-renewal in cervical cancer stem-like cells in vitro and nude mice models. World J Stem Cells 2025; 17(3): 99472
- URL: https://www.wjgnet.com/1948-0210/full/v17/i3/99472.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i3.99472