Copyright
©The Author(s) 2025.
World J Stem Cells. Mar 26, 2025; 17(3): 102067
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.102067
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.102067
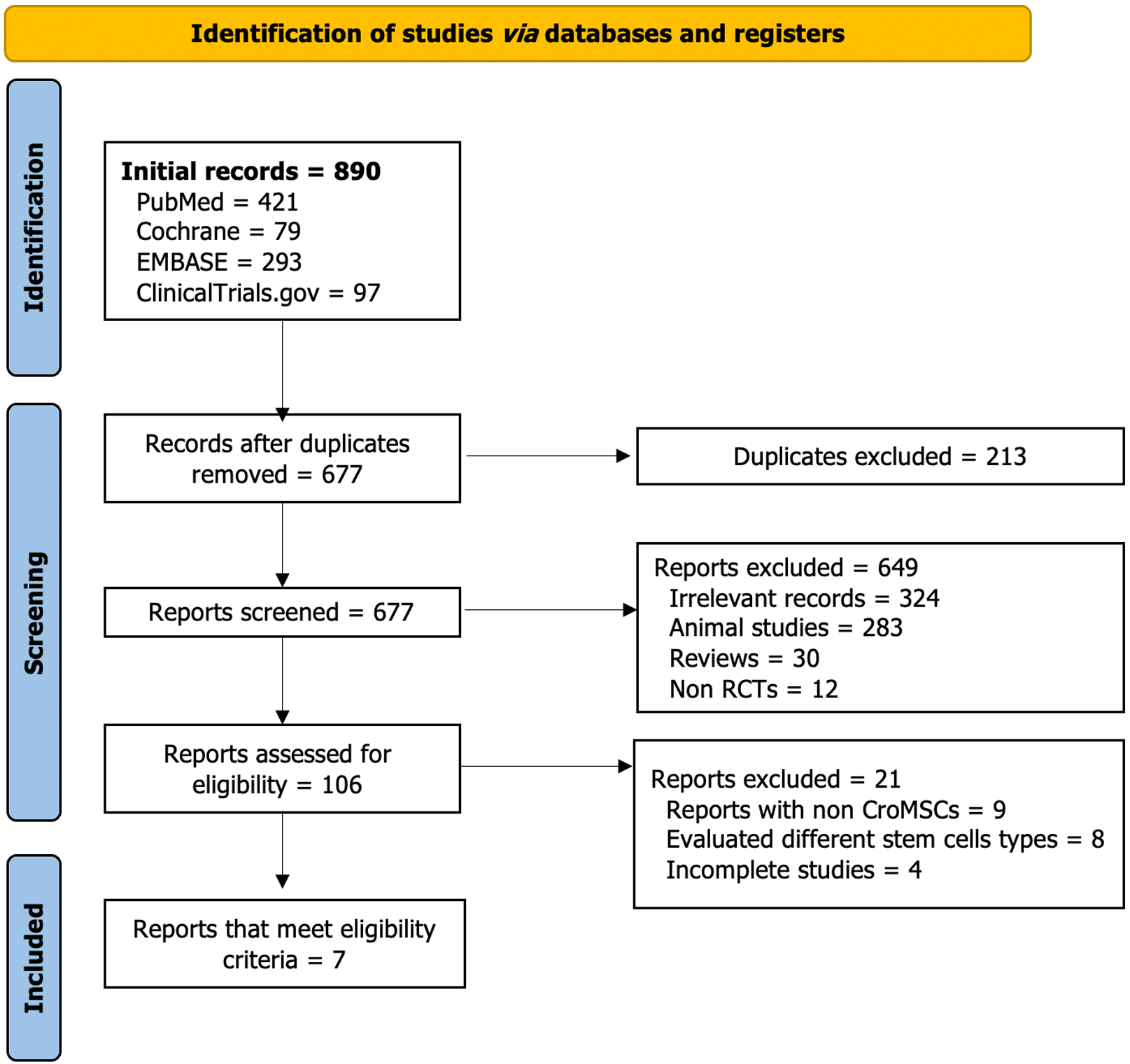
Figure 1 Study selection flow diagram (PRISMA chart).
RCTs: Randomized controlled trials; Cryo-MSCs: Cryopreserved mesenchymal stem cells.
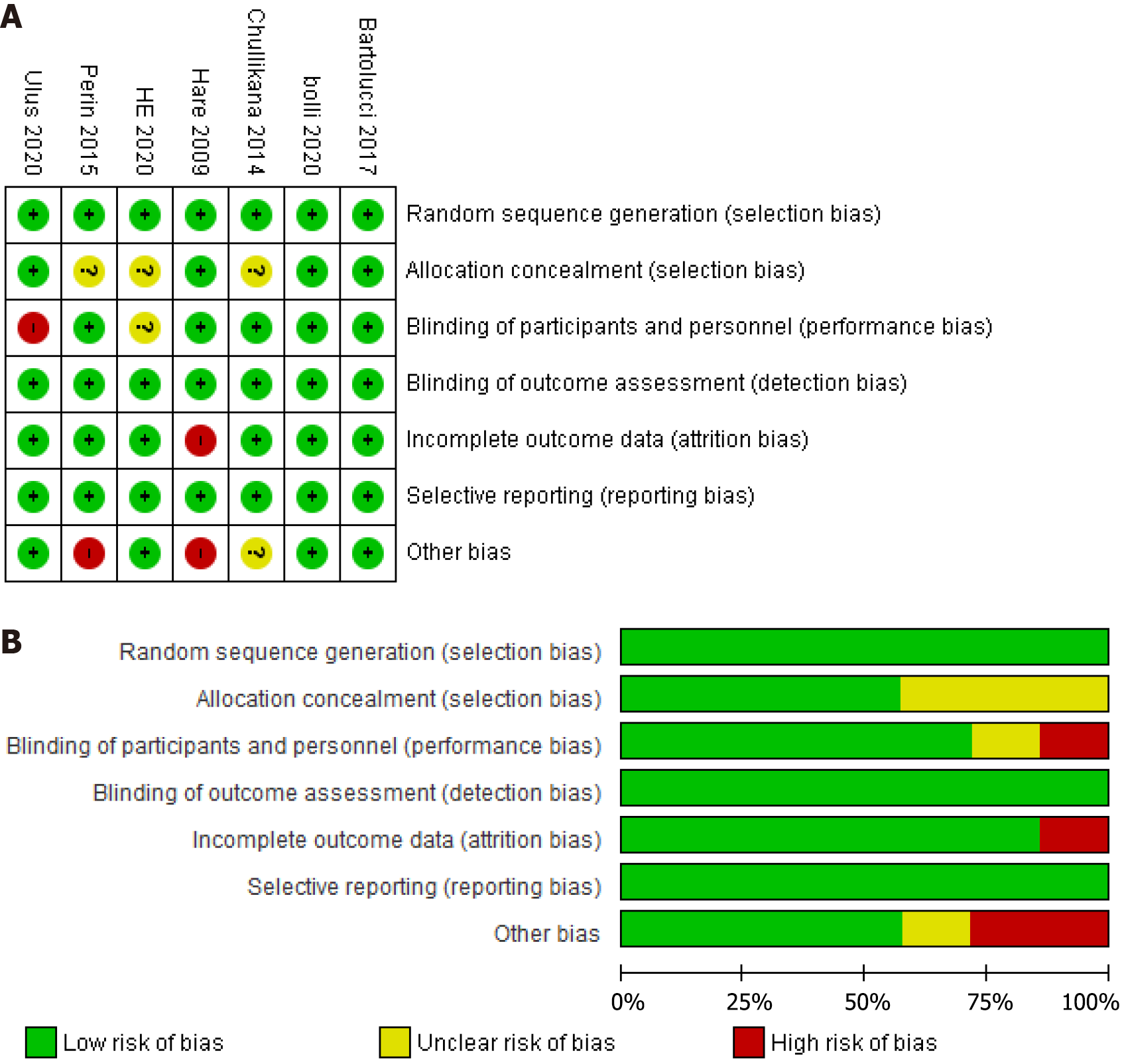
Figure 2 Risk of bias summary and graph.
A: Risk of bias summary; B: Risk of bias graph. Symbols: (+) low risk of bias, (?) unclear risk of bias, (-) high risk of bias.
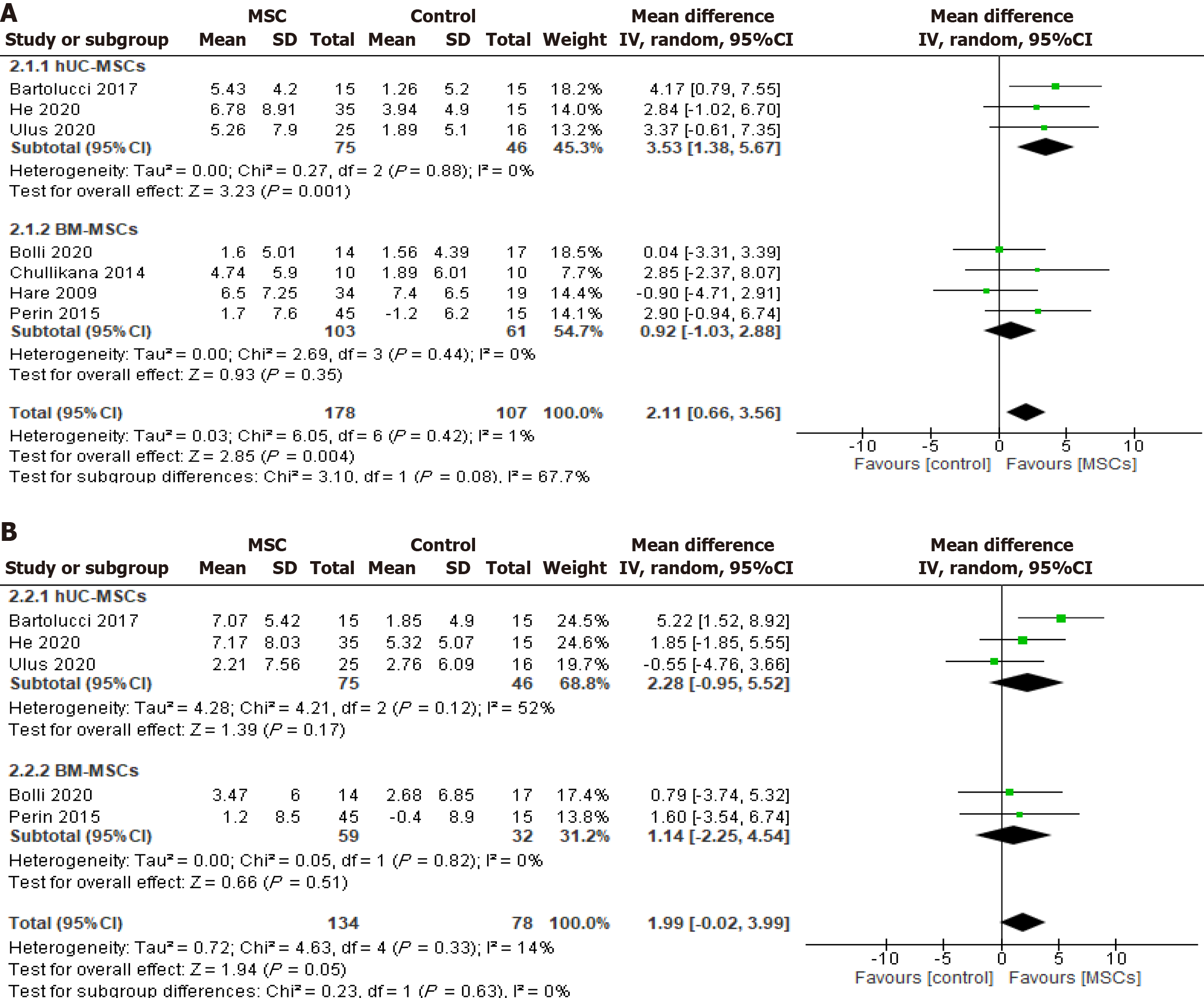
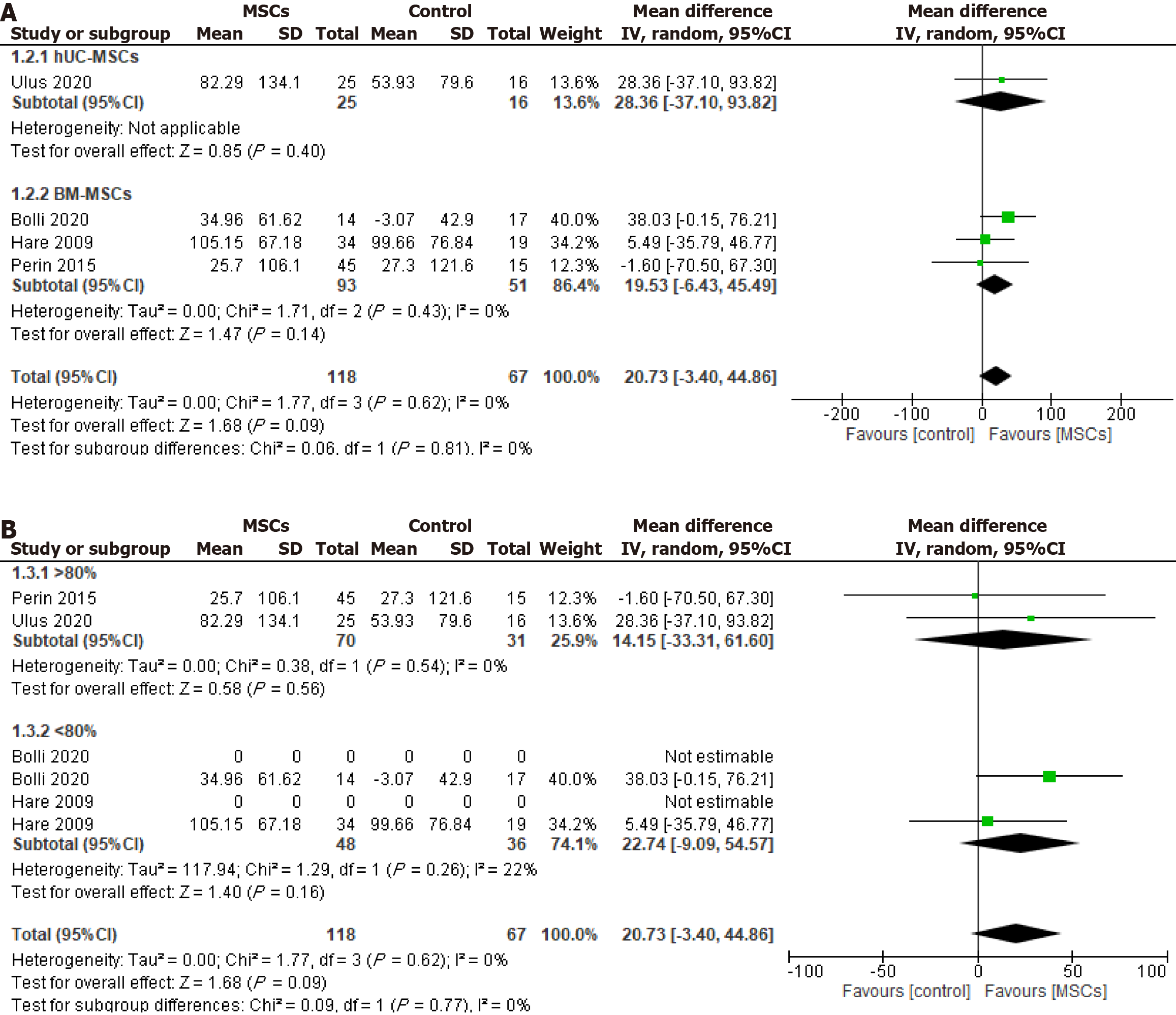
Figure 3 Effect of cryopreserved mesenchymal stem cell therapy on left ventricular ejection fraction sub-grouped according to cell source: Umbilical cord-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells.
A: Change from the baseline to six months of follow-up; B: Change from the baseline to twelve months of follow-up; hUC-MSC: Human umbilical cord-derived mesenchymal stem cell; BM-MSC: Bone marrow-derived mesenchymal stem cell.
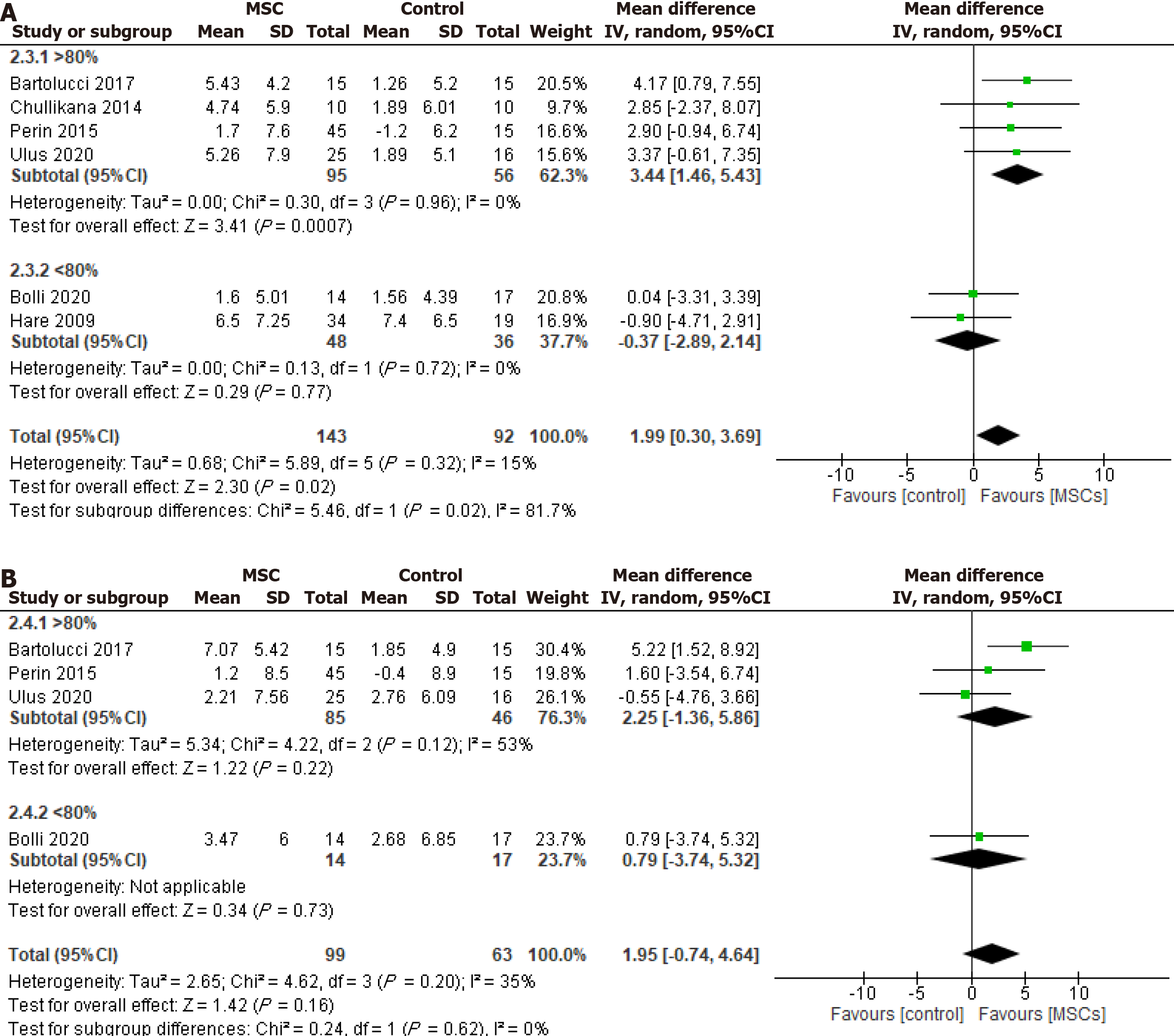
Figure 4 Effect of cryopreserved mesenchymal stem cell therapy on left ventricular ejection fraction sub-grouped according to cellular post-thaw viability as > 80% and < 80%.
A: Change from the baseline to 6 mo of follow-up; B: Change from the baseline to 12 mo of follow-up. MSC: Mesenchymal stem cell; CI: Confidence interval.
Figure 5 Effect of cryopreserved mesenchymal stem cell therapy on 6 min walk distance sub-grouped according to cell source.
A and B: Umbilical cord-derived mesenchymal stem cells (A) and bone marrow-derived mesenchymal stem cells (B) cellular post-thaw viability as > 80% and < 80%. MSC: Mesenchymal stem cell; CI: Confidence interval; hUC-MSC: Human umbilical cord-derived mesenchymal stem cell; BM-MSC: Bone marrow-derived mesenchymal stem cell.
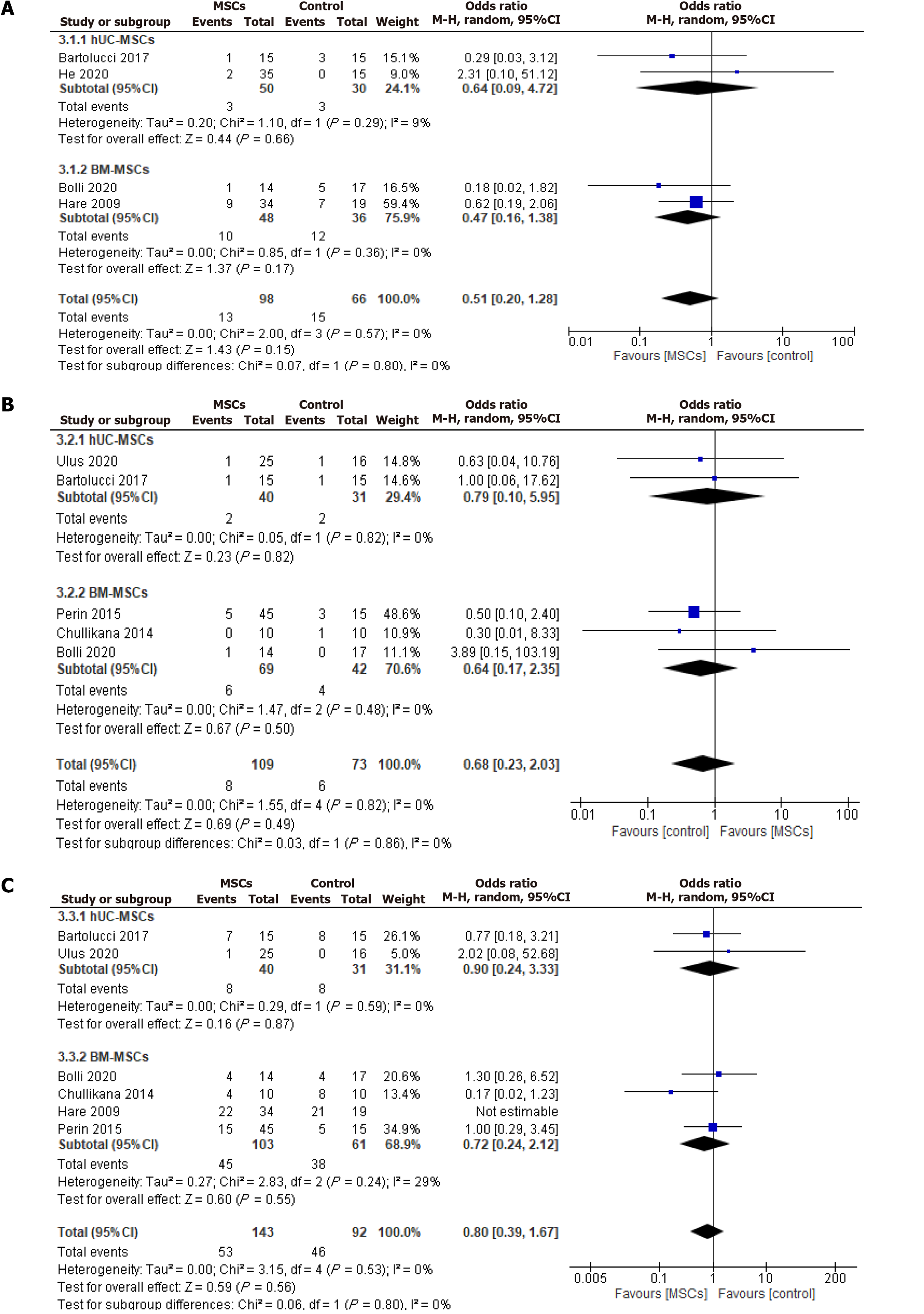
Figure 6 Odds ratio of the safety outcomes.
A: Rehospitalization; B: Mortality; C: Major adverse cardiac events. MSC: Mesenchymal stem cell; CI: Confidence interval; hUC-MSC: Human umbilical cord-derived mesenchymal stem cell; BM-MSC: Bone marrow-derived mesenchymal stem cell.
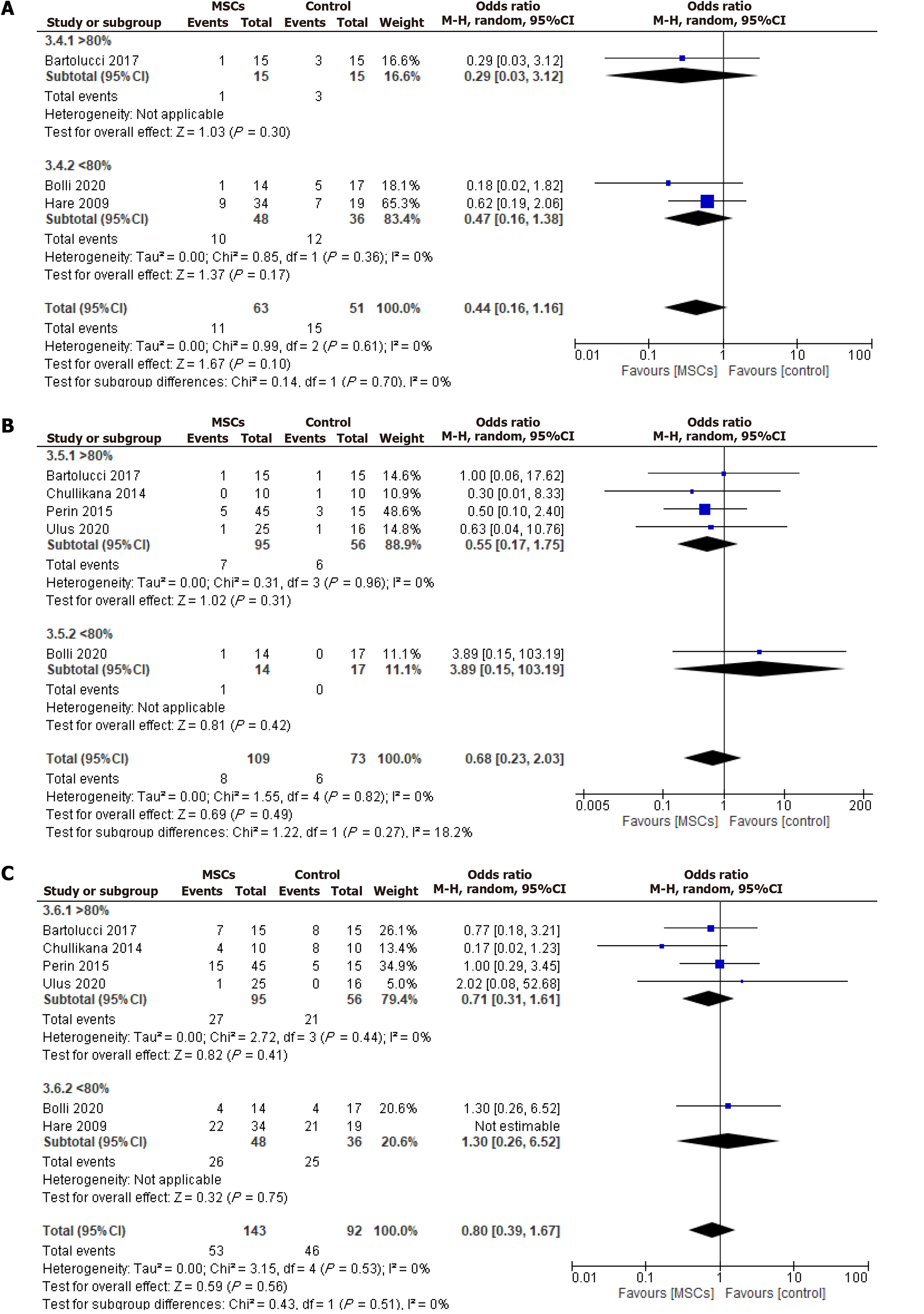
Figure 7 Odds ratio of the safety outcomes sub-grouped according to post-thaw cellular viability.
A: Rehospitalization; B: Mortality; C: Major adverse cardiac events. MSC: Mesenchymal stem cell; CI: Confidence interval.
- Citation: Safwan M, Bourgleh MS, Haider KH. Clinical experience with cryopreserved mesenchymal stem cells for cardiovascular applications: A systematic review. World J Stem Cells 2025; 17(3): 102067
- URL: https://www.wjgnet.com/1948-0210/full/v17/i3/102067.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i3.102067