Copyright
©The Author(s) 2025.
World J Stem Cells. Mar 26, 2025; 17(3): 101454
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.101454
Published online Mar 26, 2025. doi: 10.4252/wjsc.v17.i3.101454
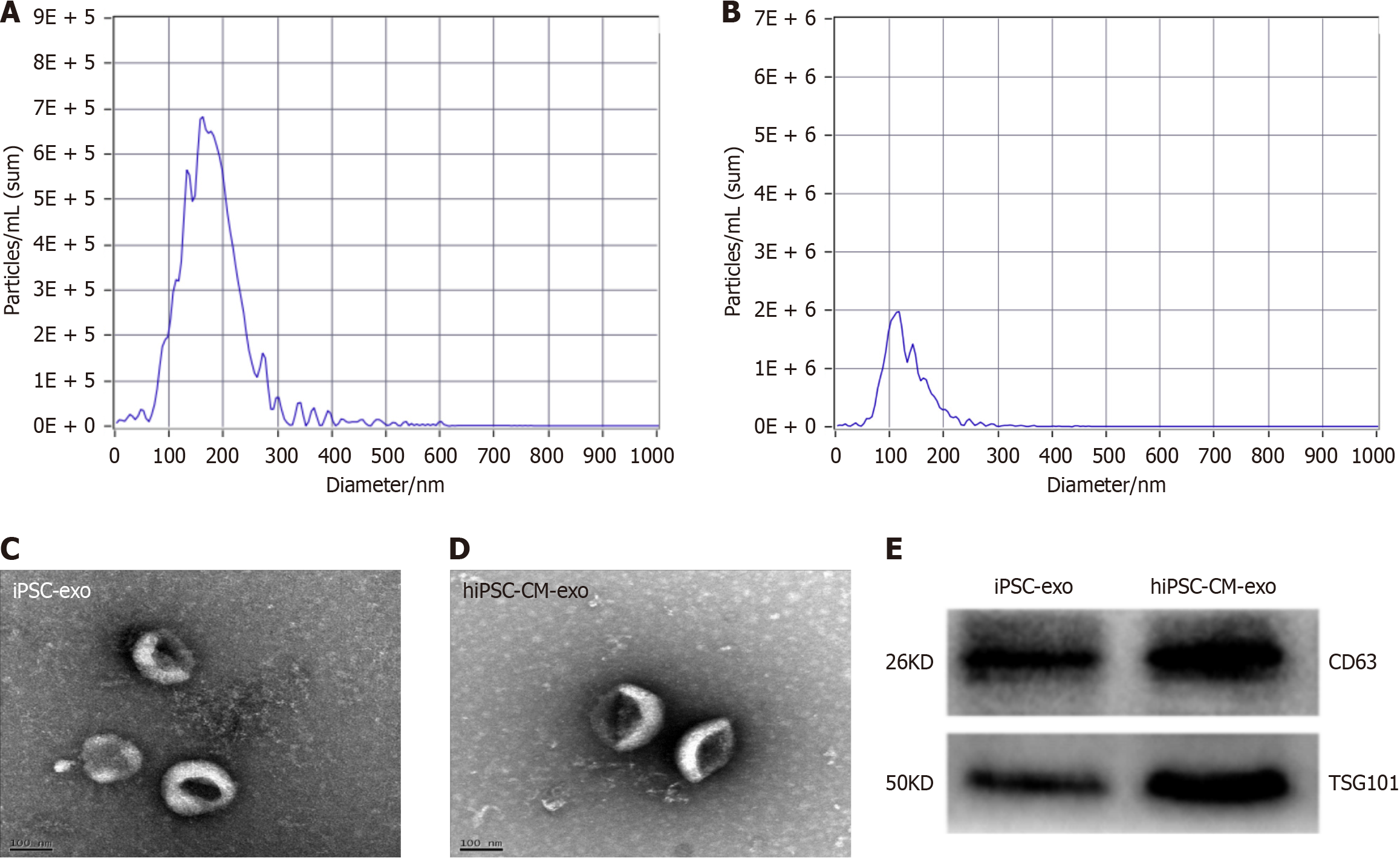
Figure 1 Characterization of human-induced pluripotent stem cells and human-induced pluripotent stem cell-derived cardiomyocyte exosomes.
A and B: The size was assessed utilizing nanoparticle tracking analysis; C and D: Morphology of human-induced pluripotent stem cells (hiPSCs) and human-induced pluripotent SC-derived cardiomyocyte exosomes (hiPSC-CM-exos) was analyzed via electron microscopy; E: Western blot analysis confirmed the expression of cluster of differentiation 63 (CD63) and tumor susceptibility gene 101 in exos derived from hiPSCs and hiPSC-CMs. iPSC-exos: Induced pluripotent stem cell-derived exosomes.
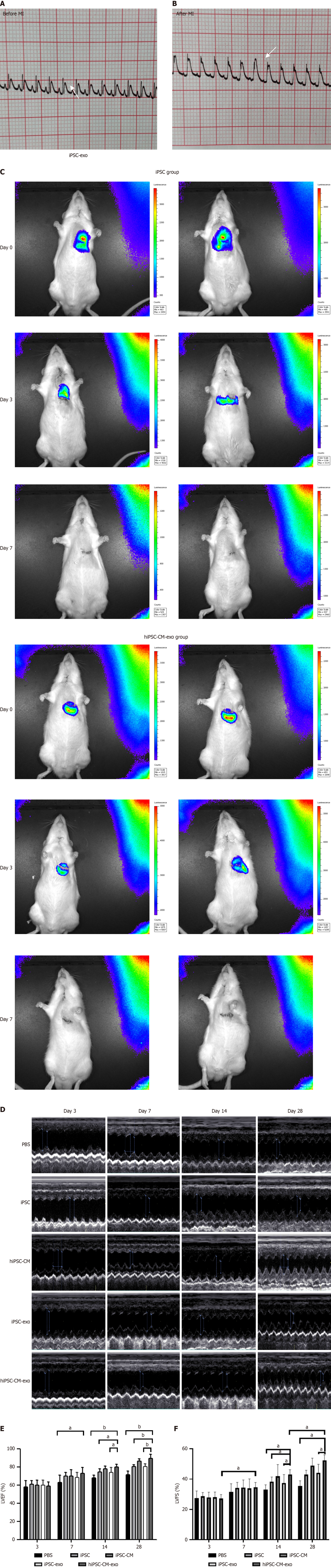
Figure 2 Human-induced pluripotent stem cell-derived cardiomyocyte exosomes improve cardiac function in a murine myocardial infarction model.
A and B: Electrocardiogram changes after left anterior descending following left anterior descending ligation; all rats displayed ST segment elevations (indicated by the white arrow); C: Bioluminescence imaging of induced pluripotent stem cells (iPSCs) and human-induced pluripotent SC-derived cardiomyocytes (hiPSC-CMs) on days 0, 3, and 7 post-cell transplantation; D: Left ventricular ejection fractions (LVEFs) were assessed on days 3, 7, 14, and 28 post-myocardial infarction (MI) injury and treatment. Data are shown as the mean ± SD from n = 7 biologically independent samples across different groups; E and F: LVEF (E) and LVFS (F) were measured on days 3, 7, 14, and 28 post-MI injury and treatment. Data are shown as the mean ± SD from n = 7 biologically independent samples across different groups. aP < 0.05, bP < 0.05. hiPSC-CM-exos: Human-induced pluripotent stem cell-derived cardiomyocyte-derived exosomes; iPSC-CMs: Induced pluripotent stem cell-derived cardiomyocytes; iPSC-exos: Induced pluripotent stem cell-derived exosomes; PBS: Phosphate-buffered saline.
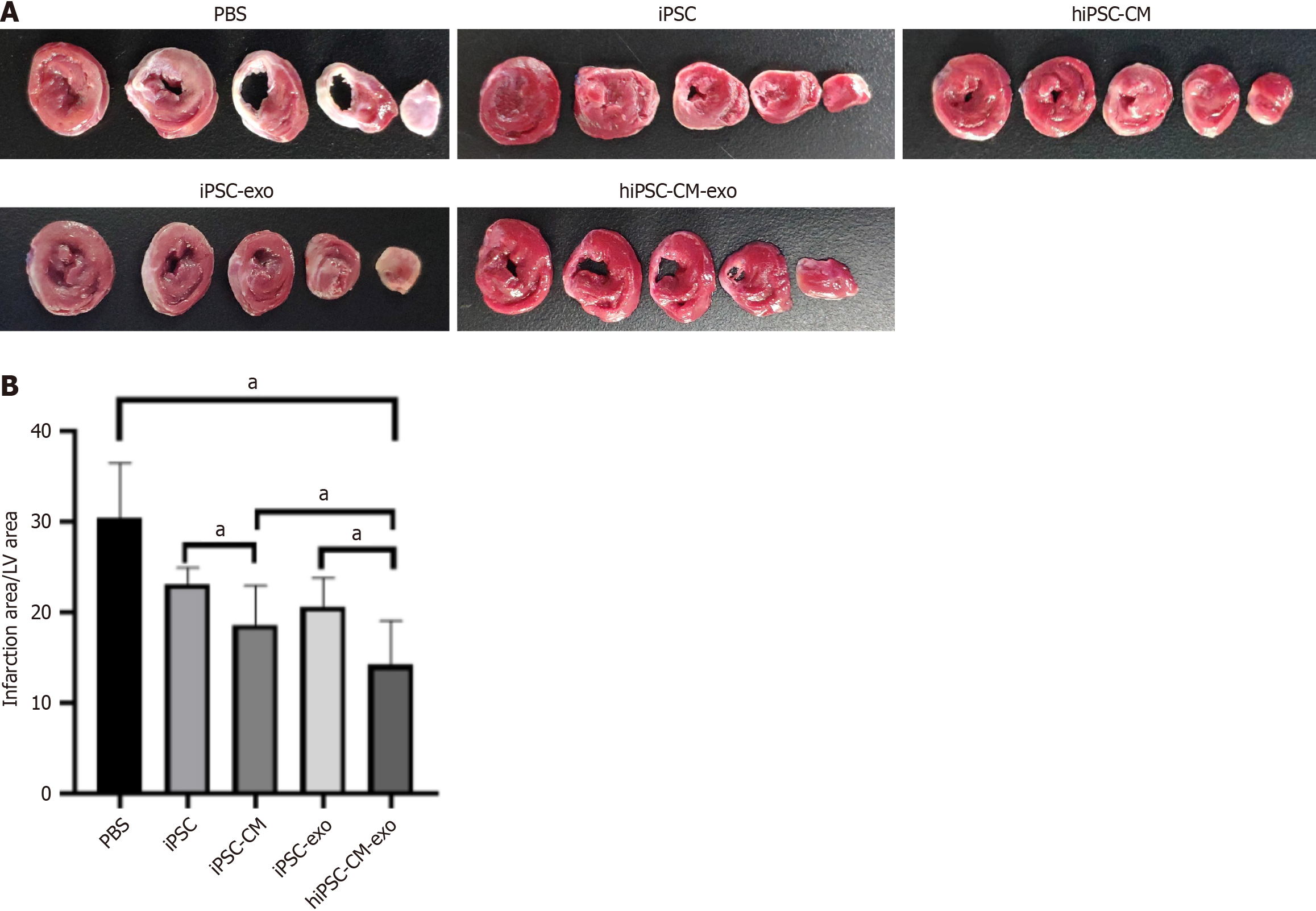
Figure 3 Human-induced pluripotent stem cell-derived cardiomyocyte exosomes reduce infarction area.
A: Representative images of 2,3,5-triphenyltetrazoliumchloride staining across five continuous slices of the left ventricle from various group hearts; B: The infarct size, which is expressed as a percentage of the total left ventricle area, is depicted. Data are shown as the mean ± SD from n = 3 biologically independent samples among different groups. aP < 0.05. hiPSC-CMs: Human-induced pluripotent stem cell-derived cardiomyocytes; hiPSC-CM-exos: Human-induced pluripotent stem cell-derived cardiomyocyte-derived exosomes; iPSCs: Induced pluripotent stem cells; iPSC-CMs: Induced pluripotent stem cell-derived cardiomyocytes; iPSC-exos: Induced pluripotent stem cell-derived exosomes; LV: Left ventricular; PBS: Phosphate-buffered saline.
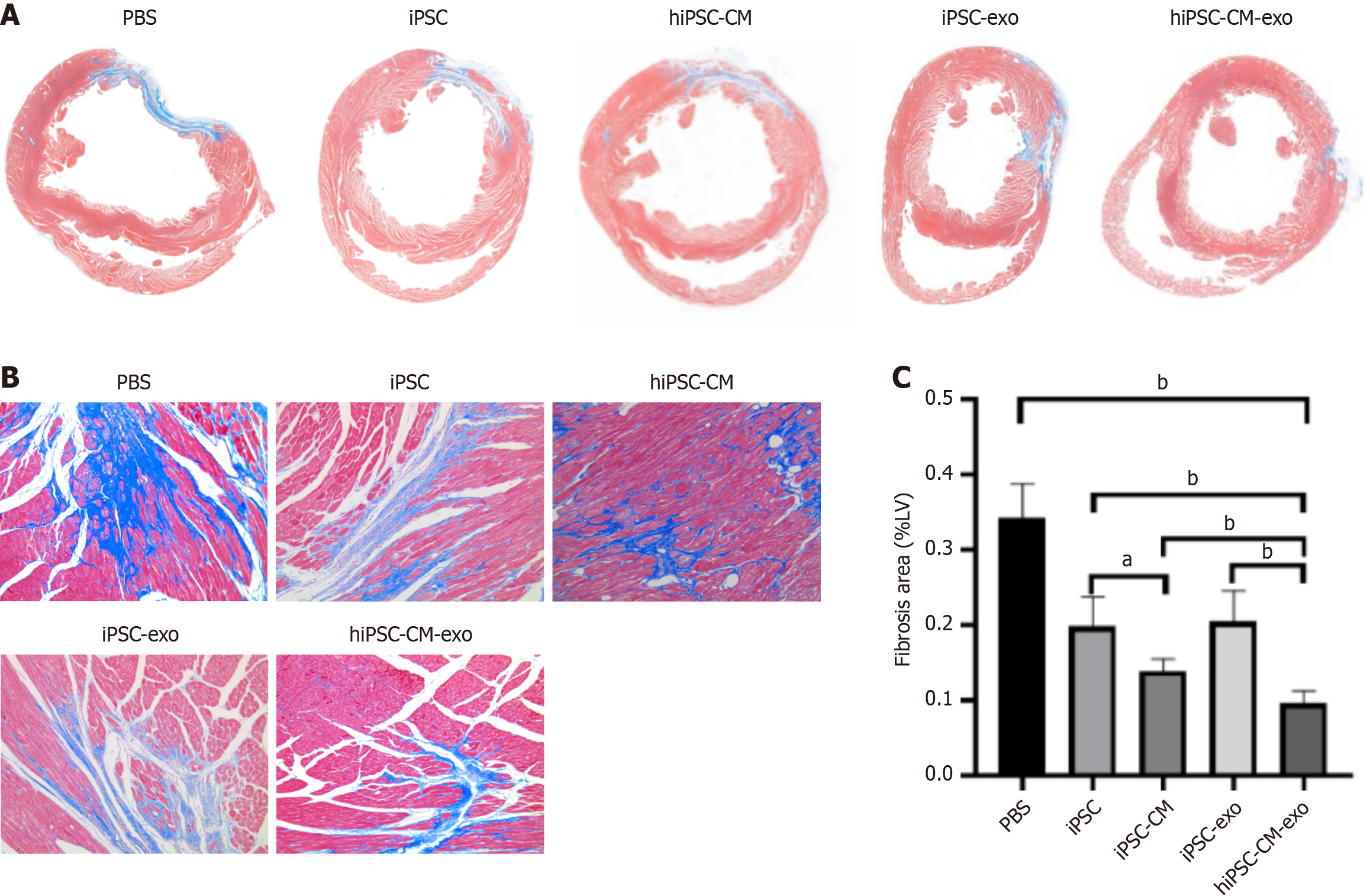
Figure 4 Human-induced pluripotent stem cell-derived cardiomyocytes exosomes reduce myocardial fibrosis.
A: Representative images of Masson trichrome staining from various groups are presented in the upper panel, with a high magnification view shown in the lower panel (100 ×); B and C: The Masson average optical density per area was used as an indicator of the cardiac fibrosis level. Data are shown as the mean ± SD from n = 4 biologically independent samples across different groups. aP < 0.05; bP < 0.01. hiPSC-CMs: Human-induced pluripotent stem cell-derived cardiomyocytes; hiPSC-CM-exos: Human-induced pluripotent stem cell-derived cardiomyocyte-derived exosomes; iPSCs: Induced pluripotent stem cells; iPSC-CMs: Induced pluripotent stem cell-derived cardiomyocytes; iPSC-exos: Induced pluripotent stem cell-derived exosomes; LV: Left ventricular; PBS: Phosphate-buffered saline.
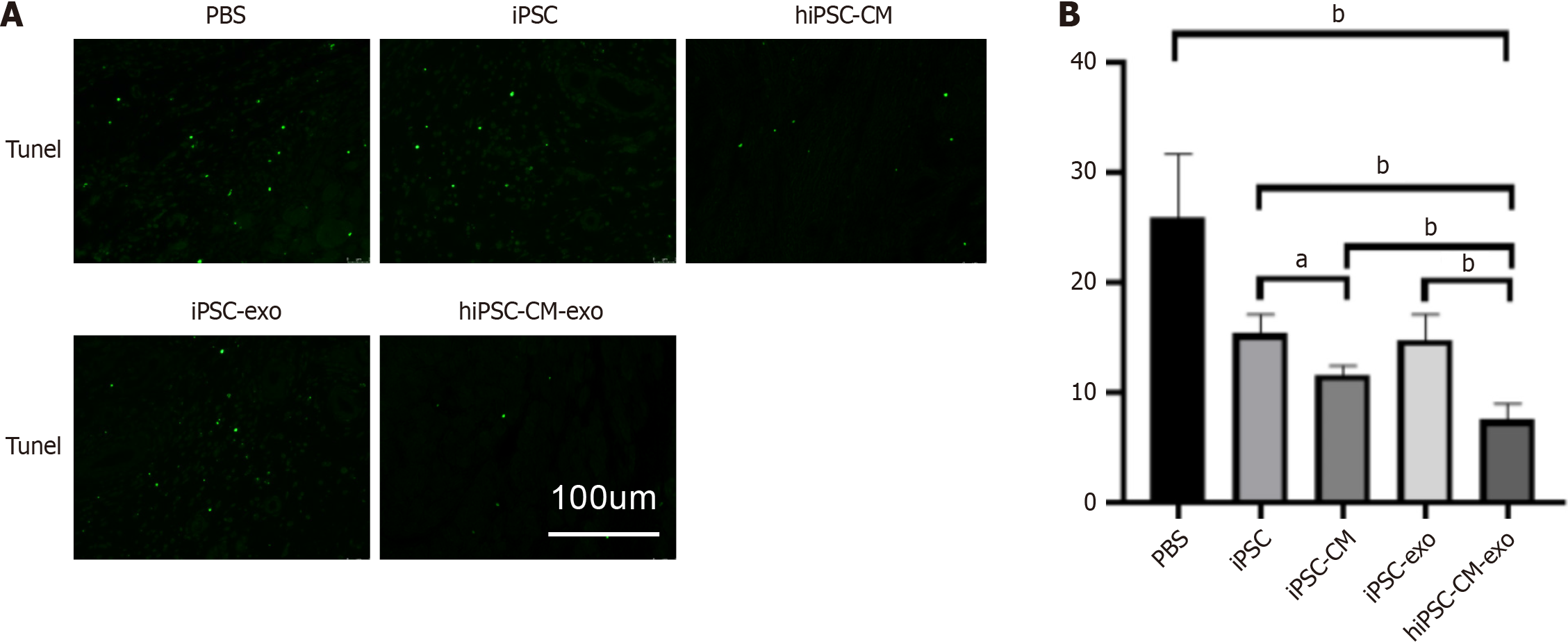
Figure 5 Human-induced pluripotent stem cell-derived cardiomyocyte exosomes reduce apoptosis when administered to infarcted rat hearts.
A: Apoptotic cells were identified in sections from the border zone of infarcted hearts from animals across various groups using the transferase dUTP nick-end labeling assay; B: Apoptosis was quantified as the percentage of cells positive for transferase dUTP nick-end labeling staining. Data are shown as the mean ± SD from n = 3 biologically independent samples among different groups. aP < 0.05, bP < 0.01. hiPSC-CMs: Human-induced pluripotent stem cell-derived cardiomyocytes; hiPSC-CM-exos: Human-induced pluripotent stem cell-derived cardiomyocyte-derived exosomes; iPSCs: Induced pluripotent stem cells; iPSC-CMs: Induced pluripotent stem cell-derived cardiomyocytes; iPSC-exos: Induced pluripotent stem cell-derived exosomes; PBS: Phosphate-buffered saline; TUNEL: Transferase dUTP nick-end labeling.
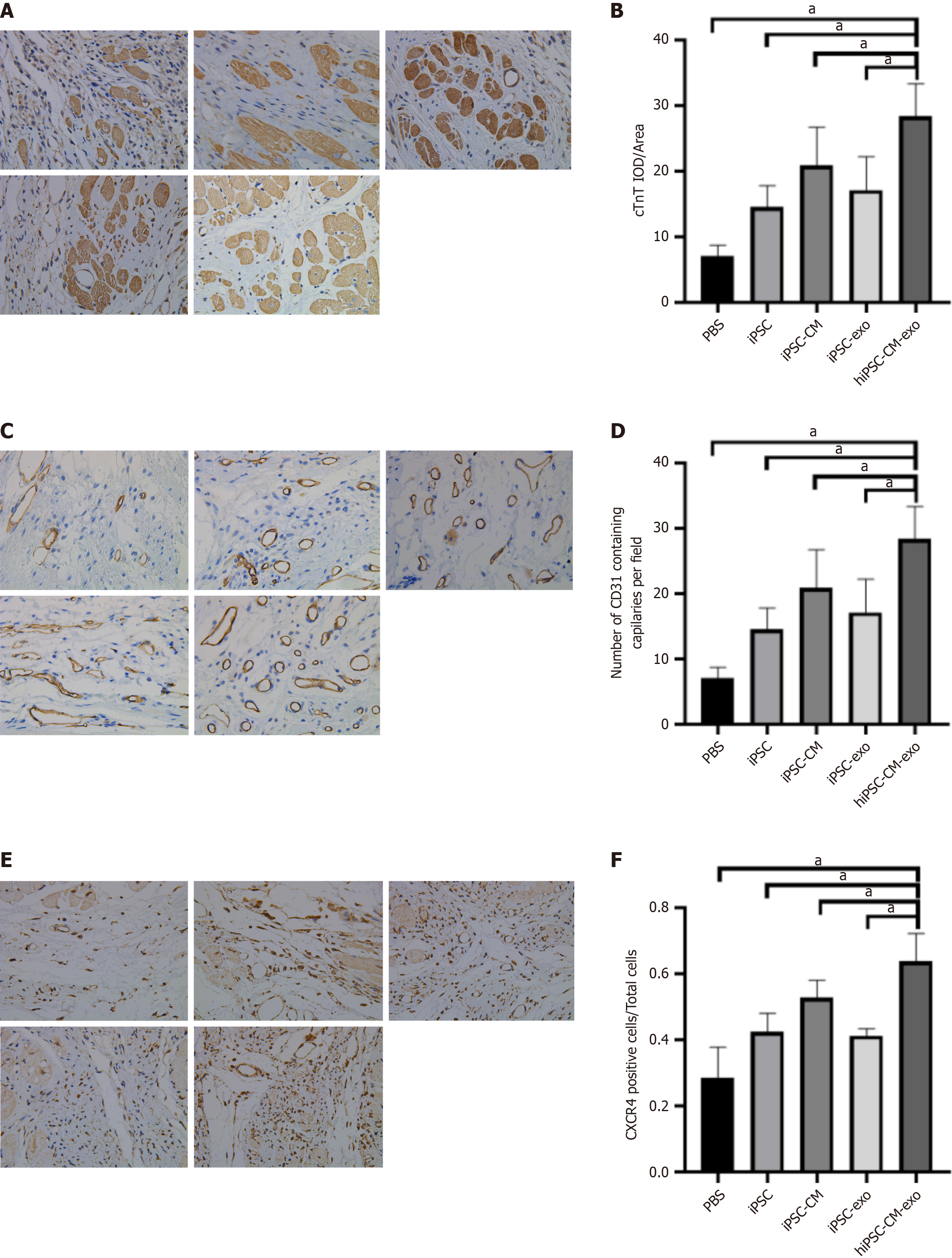
Figure 6 Administration of human-induced pluripotent stem cell-derived cardiomyocytes exosomes to infarcted rat hearts enhances the expression of cardiac troponin T, cluster of differentiation 31, and C-X-C chemokine receptor type 4.
A: Post-myocardial infarction (MI) border zone sections were subjected to immunohistochemistry for cardiac troponin T (cTNT) expression. The black arrow indicates the cTNT-positive expression region; B: Average optical density value was used to represent the level of cTNT expression; C: Four weeks after MI, BZ sections were immunohistochemically stained for cluster of differentiation 31 (CD31). The black arrow indicates the CD31-positive expression region; D: Number of CD31-positive blood vessels serve as an indicator of angiogenesis at the myocardial infarction edge; E: BZ sections obtained 4 weeks after MI were immunohistochemically analyzed for C-X-C chemokine receptor 4 (CXCR4) expression. The black arrow indicated the CXCR4-positive expression region; F: Ratio of CXCR4-positive cells to total cells reflects the level of CXCR4 expression. Data are shown as the mean ± SD from n = 4 biologically independent samples across different groups. aP < 0.05. hiPSC-CM-exos: Human-induced pluripotent stem cell-derived cardiomyocyte-derived exosomes; iPSCs: Induced pluripotent stem cells; iPSC-CMs: Induced pluripotent stem cell-derived cardiomyocytes; iPSC-exos: Induced pluripotent stem cell-derived exosomes; PBS: Phosphate-buffered saline.
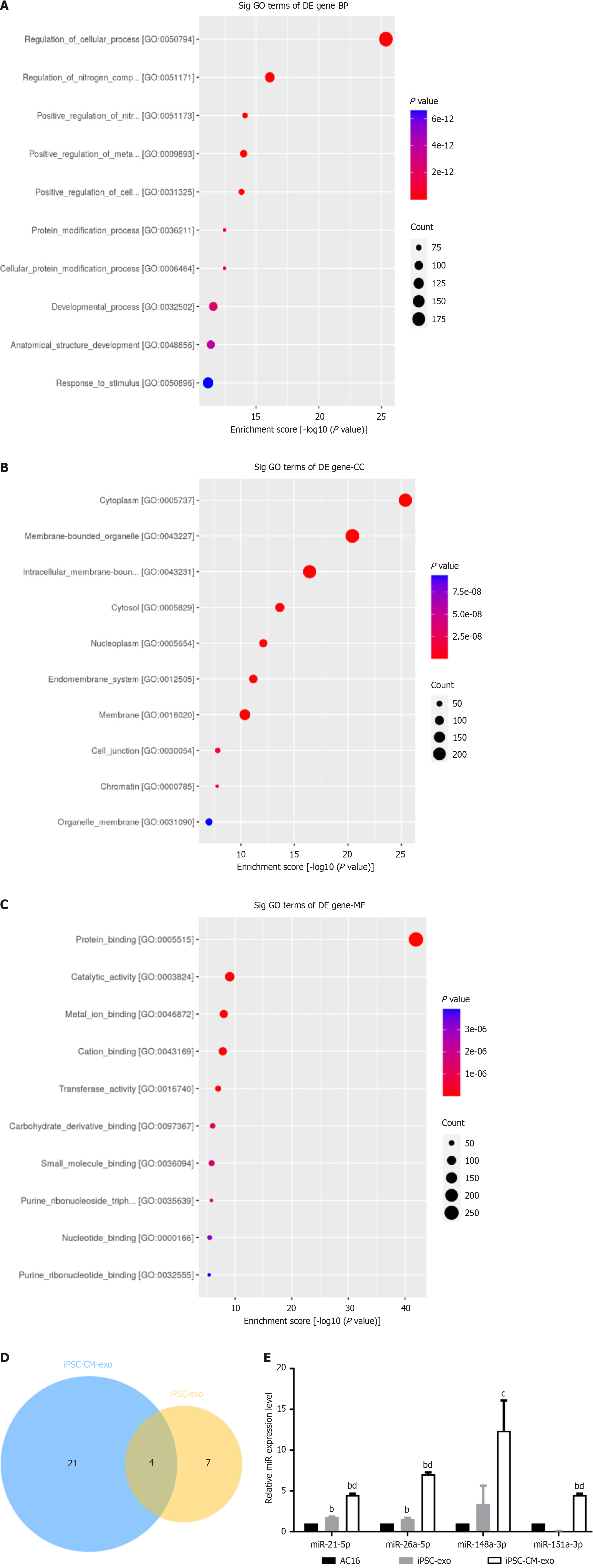
Figure 7 Data analysis of sequencing microRNA.
A: Enrichment result of biological processes (BP) is Gene Ontology (GO): 0050794 regulation of cellular processes; GO: 0051171 regulation of nitrogen compound metabolic process; GO: 0051173 positive regulation of nitrogen compound metabolic process; B: Enrichment results of cellular components (CC) are GO: 0005737 cytoplasm, GO: 0043227 membrane bound organelle, GO: 0043231 intracellular membrane bound organelle; C: Molecular function (MF) enrichment results are GO: 0005515 protein binding, GO: 0003824 catalytic activity, and GO: 0046872 metal ion binding; D: Four miRNAs hsa-miR-148a-3p, hsa-miR-21-5p, hsa-miR-151a-3p and hsa-miR-26a-5p, were obtained from the collection of highly expressed microRNAs in human-induced pluripotent stem cell (hiPSC)-cardiomyocyte (CM)-exosome (exo) and iPSC exo; E: MicroRNA expression in AC16, hiPSC-exo, hiPSC-CM-exo, bP < 0.01 comparison with AC16-exo, cP < 0.05 comparison with hiPSC-exo, dP < 0.01 comparison with hiPSC-exo.
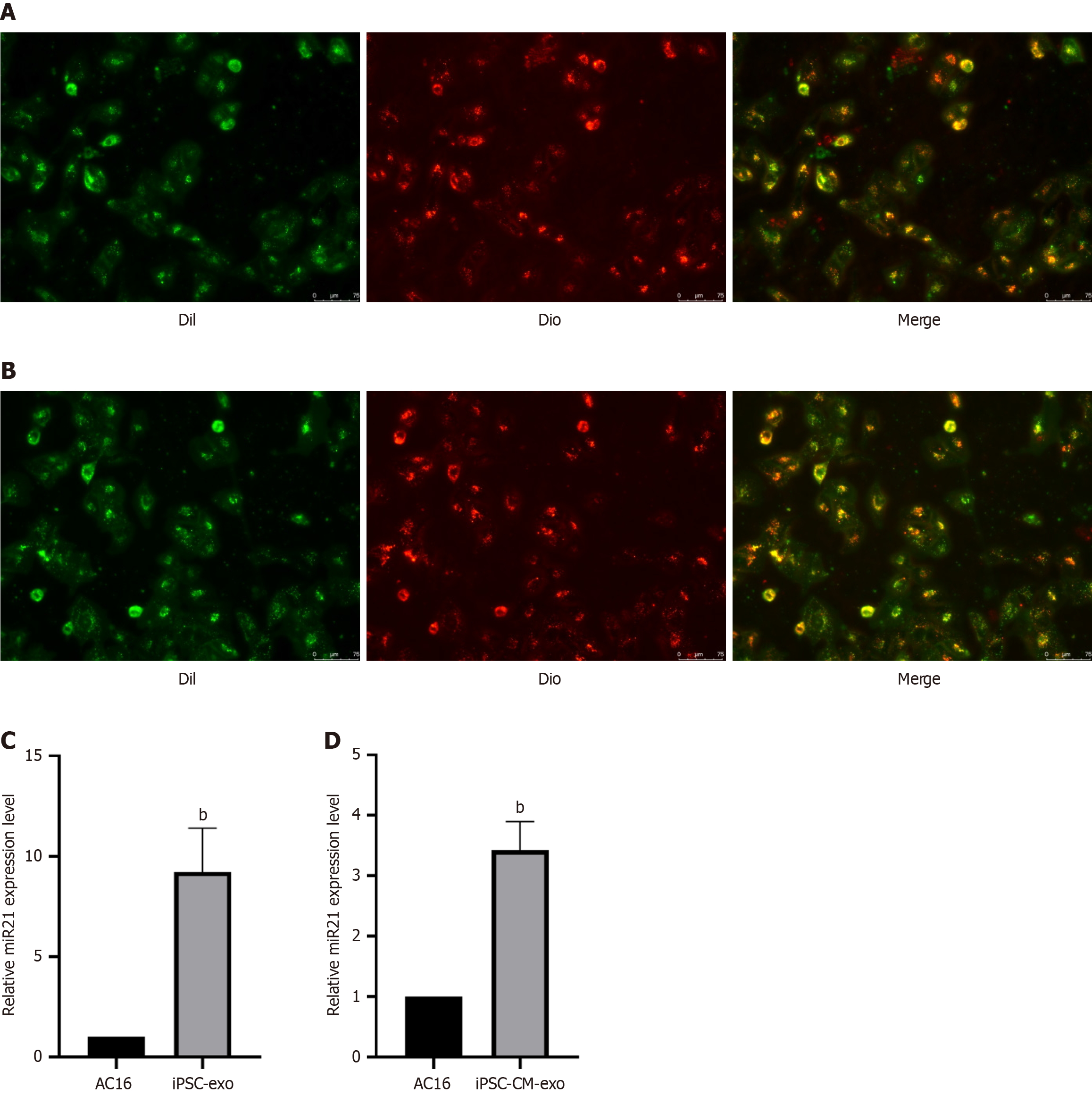
Figure 8 Effects of exosomes applied to AC16 cells on the expression level of microRNA 21.
A: AC16 cells absorb human-induced pluripotent stem cell-exosomes (hiPSC-exos). Scale bar = 75 μm; B: AC16 cells absorb hiPSC-cardiomyocyte (CM)-exos Scale bar = 75 μm; C: Expression of miR-21 in AC16 cells after absorption of iPSC-exos; D: Expression of microRNA 21 in AC16 cells after absorption of hiPSC-CM-exos, bP < 0.01.
- Citation: Jin JJ, Liu RH, Chen JY, Wang K, Han JY, Nie DS, Gong YQ, Lin B, Weng GX. MiR-21-5p-enriched exosomes from hiPSC-derived cardiomyocytes exhibit superior cardiac repair efficacy compared to hiPSC-derived exosomes in a murine MI model. World J Stem Cells 2025; 17(3): 101454
- URL: https://www.wjgnet.com/1948-0210/full/v17/i3/101454.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i3.101454