Copyright
©The Author(s) 2025.
World J Stem Cells. Feb 26, 2025; 17(2): 101395
Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.101395
Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.101395
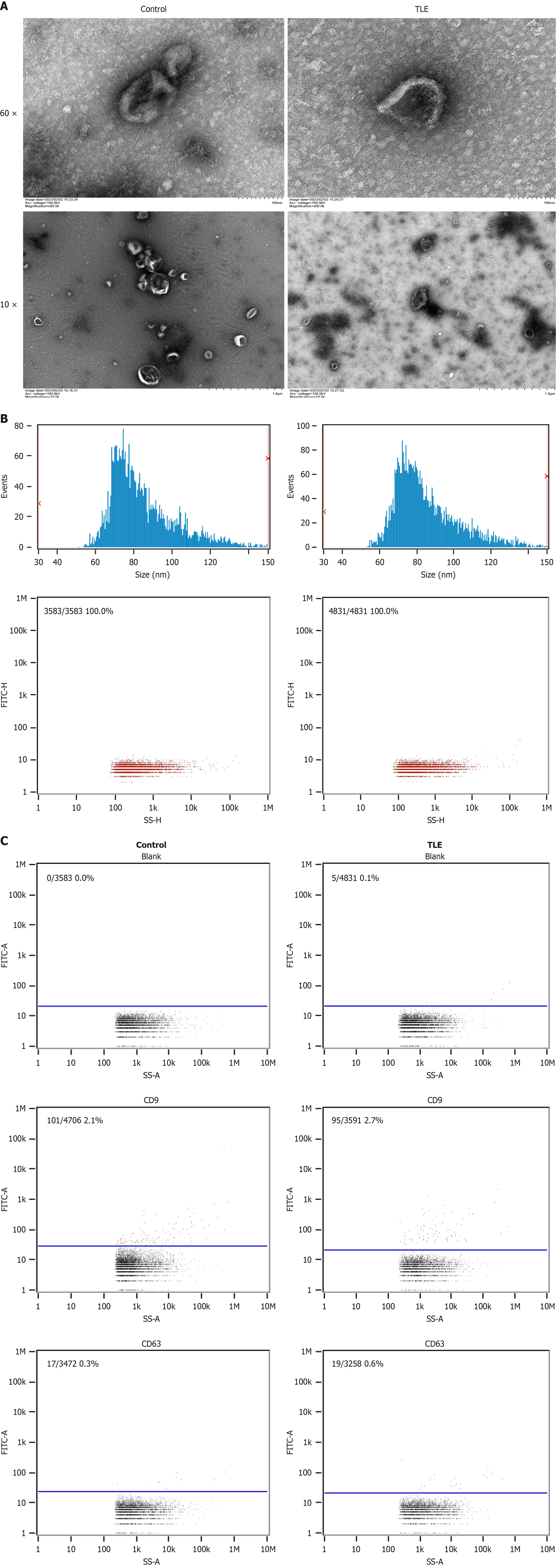
Figure 1 Characteristic of exosomes from bone marrow mesenchymal stem cells.
A: Transmission electron microscopy images reveal the characteristic “cup-shaped” morphology and clear membrane structure of exosomes from both the epilepsy and normal groups of mice; B: Nanoparticle tracking analysis shows the average particle size and concentration to be 8531 nm and 1.62E+10 particles/mL for the epilepsy group, and 84.64 nm and 3.29E+10 particles/mL for the normal group, respectively; C: Flow cytometry analysis demonstrates positive expression of the protein markers CD9 and CD63 in exosomes from both groups, with a slightly higher positive rate for CD9 in the epilepsy group than in the normal group and minimal expression of CD63 in both groups. TLE: Temporal lobe epilepsy.
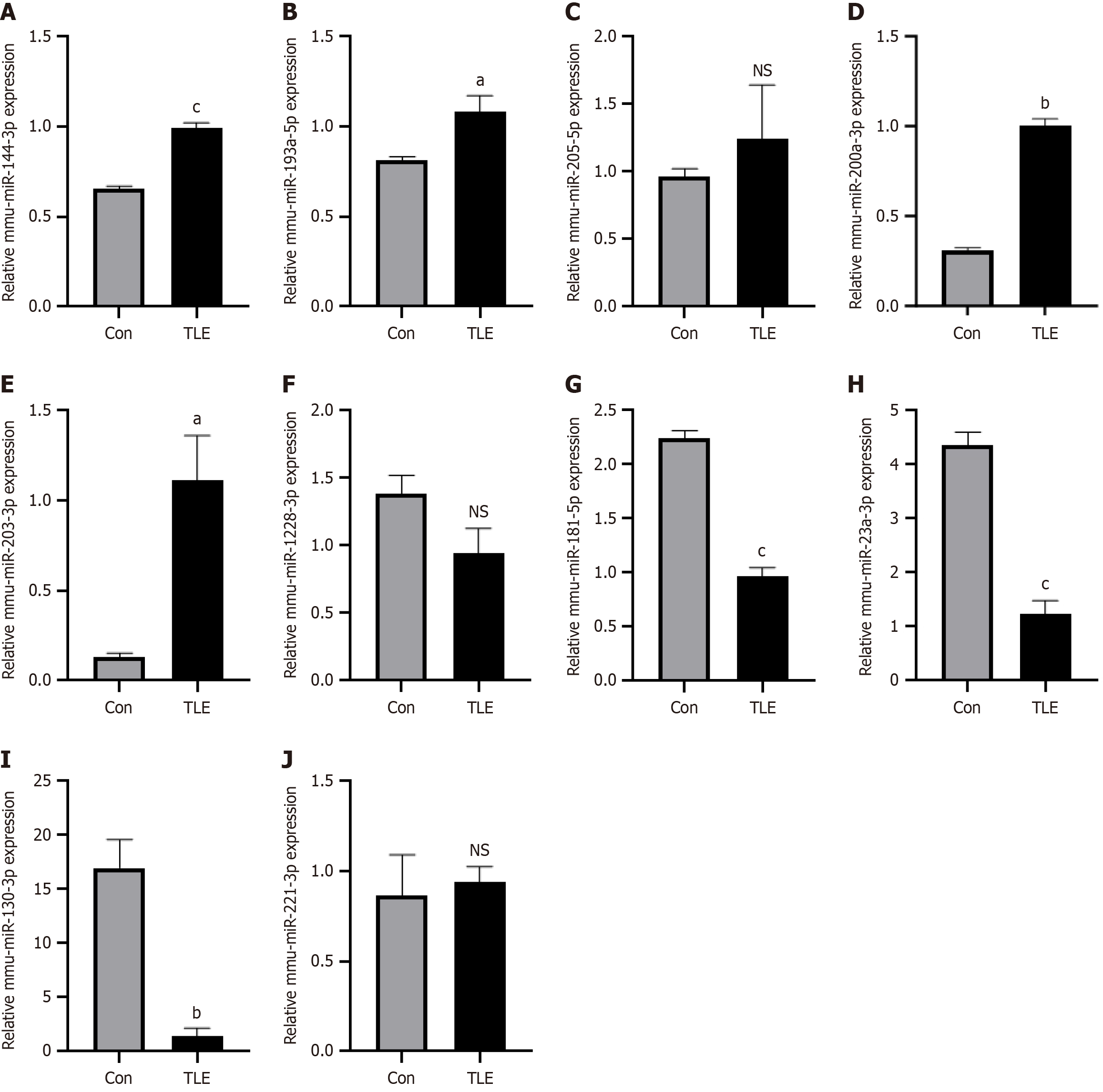
Figure 2 Quantitative polymerase chain reaction validation of DE-miRNAs.
A-J: Expression miRNAs in epileptic mouse bone marrow mesenchymal stem cells. aP < 0.05, bP < 0.01, cP < 0.001, NS: No significance. TLE: Temporal lobe epilepsy.
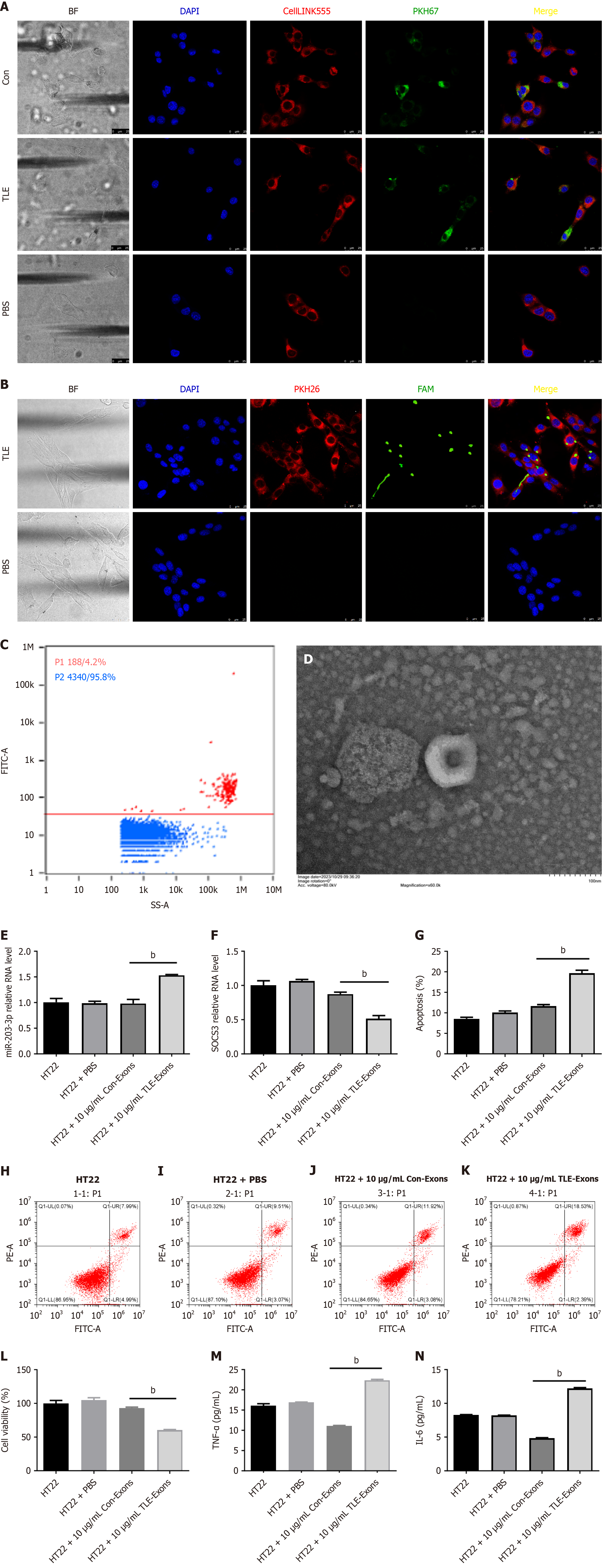
Figure 3 Exosomes secreted by bone marrow mesenchymal stem cells enhance the secretion of inflammatory mediators in hippocampal neurons.
A: Confocal microscopy images of HT22 cells co-cultured with bone marrow mesenchymal stem cell-derived exosomes. Cell nuclei were stained with DAPI, cell membranes were stained with CellLINK555, and exosomes were labeled with PKH67. The scale is 25 μm; B: Confocal microscopy imaging was performed after co-culturing HT22 cells with extracellular vesicles from bone marrow mesenchymal stem cells. DAPI staining visualized the cell nuclei, PKH26 staining labeled the cell membrane, and FAM labeling detected miR-203-3p within the extracellular vesicles. The scale bar represents 25 μm; C: Flow cytometry analysis of extracellular vesicles in the supernatant after HT22 cell transfection with FAM-miR-203-3p; D: Electron microscopic examination of extracellular vesicles in the supernatant after HT22 cell transfection with FAM-miR-203-3p; E: Quantitative polymerase chain reaction results reveal a significant increase in miR-203-3p levels in the temporal lobe epilepsy (TLE) group; F: Significant decrease in suppression of cytokine signaling 3 mRNA expression was observed in the TLE group; G-K: Flow cytometric analysis demonstrated an elevated apoptosis rate in the TLE group; L: CCK8 assay indicated reduced cell proliferation in the TLE group; M and N: Enzyme-linked immunosorbent assay detection shows upregulated levels of inflammatory factors such as tumor necrosis factor-α and interleukin-6 in the TLE group. bP < 0.01. TLE: Temporal lobe epilepsy; PBS: Phosphate-buffered saline; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
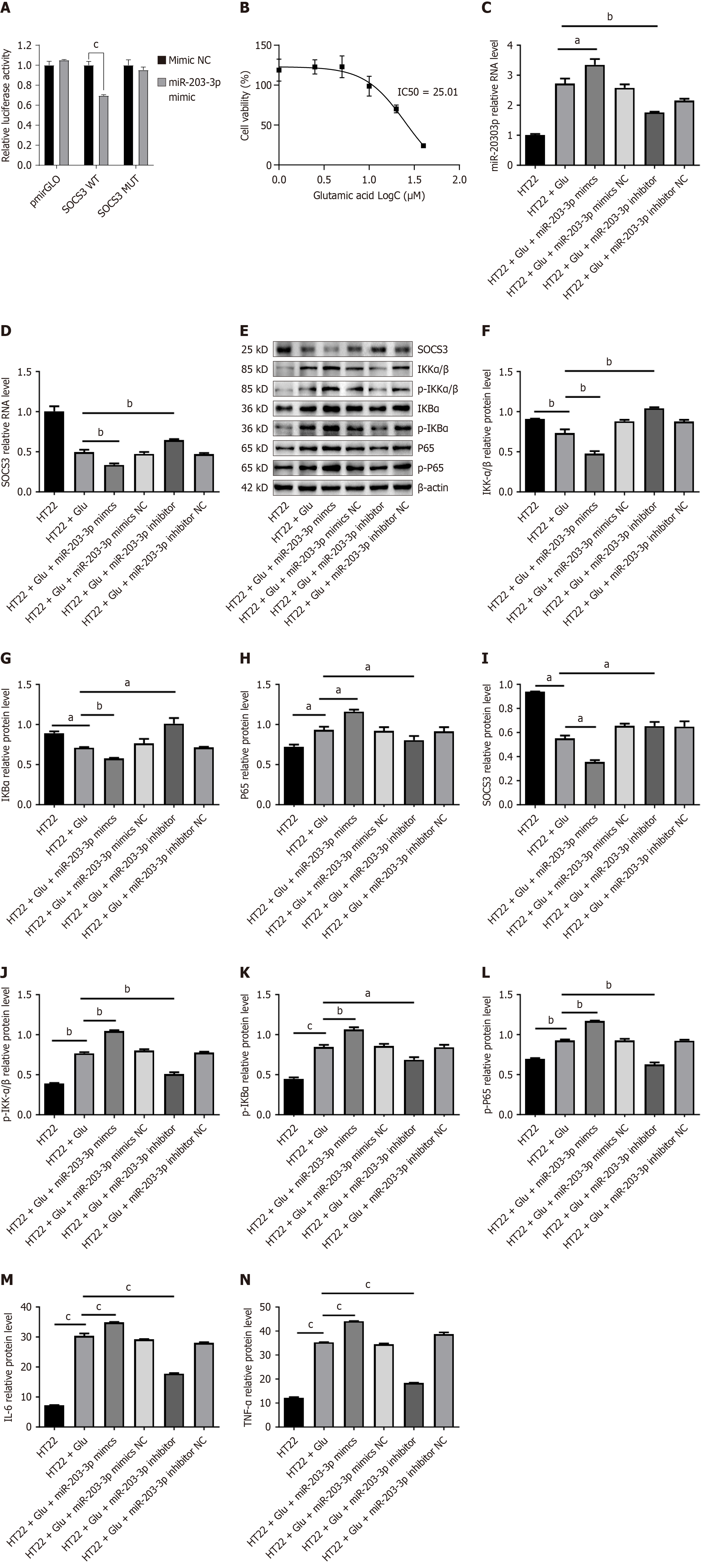
Figure 4 MiR-203-3p regulates inflammation by targeting the suppression of cytokine signaling 3-mediated nuclear factor kappaB pathway.
A: Luciferase reporter assay showing the relative firefly luciferase activity in HEK-293T cells co-transfected with wild-type or mutant suppression of cytokine signaling 3 3’ untranslated region reporter plasmids and miR-203-3p mimic or miR-203-3p; B: CCK8 assay to determine the IC50 concentration of glutamate required to induce HT22 epilepsy; C: Quantitative polymerase chain reaction analysis of miR-203-3p expression in HT22 cells after treatment with miR-203-3p mimic or inhibitor; D: Quantitative polymerase chain reaction analysis of suppression of cytokine signaling 3 mRNA expression in HT22 cells under the conditions described in Figure 3C; E-L: Western blot analysis of key proteins involved in the nuclear factor kappaB pathway, including total and phosphorylated forms of inhibitor of kappa B kinase alpha/beta, nuclear factor kappaB inhibitor alpha, and p65, in HT22 cells treated with miR-203-3p mimics or inhibitors; M and N: Enzyme-linked immunosorbent assay measurements of the inflammatory cytokines interleukin-6 and tumor necrosis factor-α in the supernatant of HT22 cells treated as in Figure 3C. Data are presented as mean ± SEM of three independent experiments. aP < 0.05, bP < 0.01, cP < 0.001. SOCS3: Suppression of cytokine signaling 3; IKKα/β: Inhibitor of kappa B kinase alpha/beta; p-IKKα/β: Phospho-inhibitor of kappa B kinase alpha/beta; IKBα: Nuclear factor kappaB inhibitor alpha; p-IKBα: Phospho-nuclear factor kappaB inhibitor alpha; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
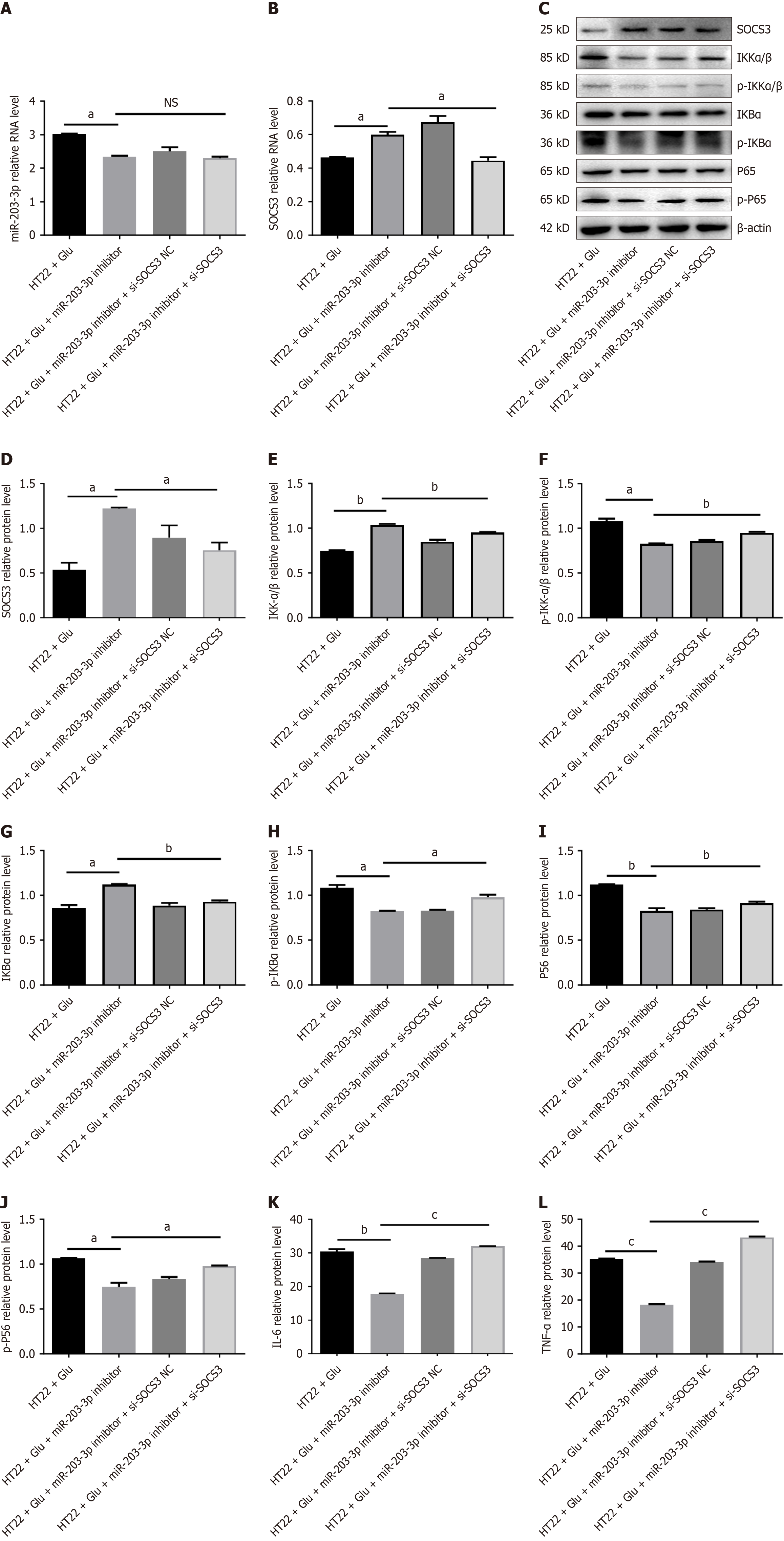
Figure 5 Downregulation of miR-203-3p and suppression of cytokine signaling 3 can reinstate an inflammatory phenotype.
A: Schematic representation of the experimental design of HT22 cells co-transfected with the miR-203-3p inhibitor and si-suppression of cytokine signaling 3 (SOCS3) or si-NC; B: Quantitative polymerase chain reaction analysis of SOCS3 mRNA expression in HT22 cells following co-transfection; C and D: Western blot analysis of SOCS3 protein levels in HT22 cells under the same conditions as Figure 5C; E-J: Western blot analysis of nuclear factor kappaB pathway markers in HT22 cells co-transfected with miR-203-3p inhibitor and si-SOCS3 or si-NC; K and L: Enzyme-linked immunosorbent assay measurements of interleukin-6 and tumor necrosis factor-α levels in the supernatant of HT22 cells co-transfected as in Figure 5C. Data are presented as mean ± SEM of three independent experiments. aP < 0.05, bP < 0.01, cP < 0.001, NS: No significance. SOCS3: Suppression of cytokine signaling 3; IKKα/β: Inhibitor of kappa B kinase alpha/beta; p-IKKα/β: Phospho-inhibitor of kappa B kinase alpha/beta; IKBα: Nuclear factor kappaB inhibitor alpha; p-IKBα: Phospho-nuclear factor kappaB inhibitor alpha; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
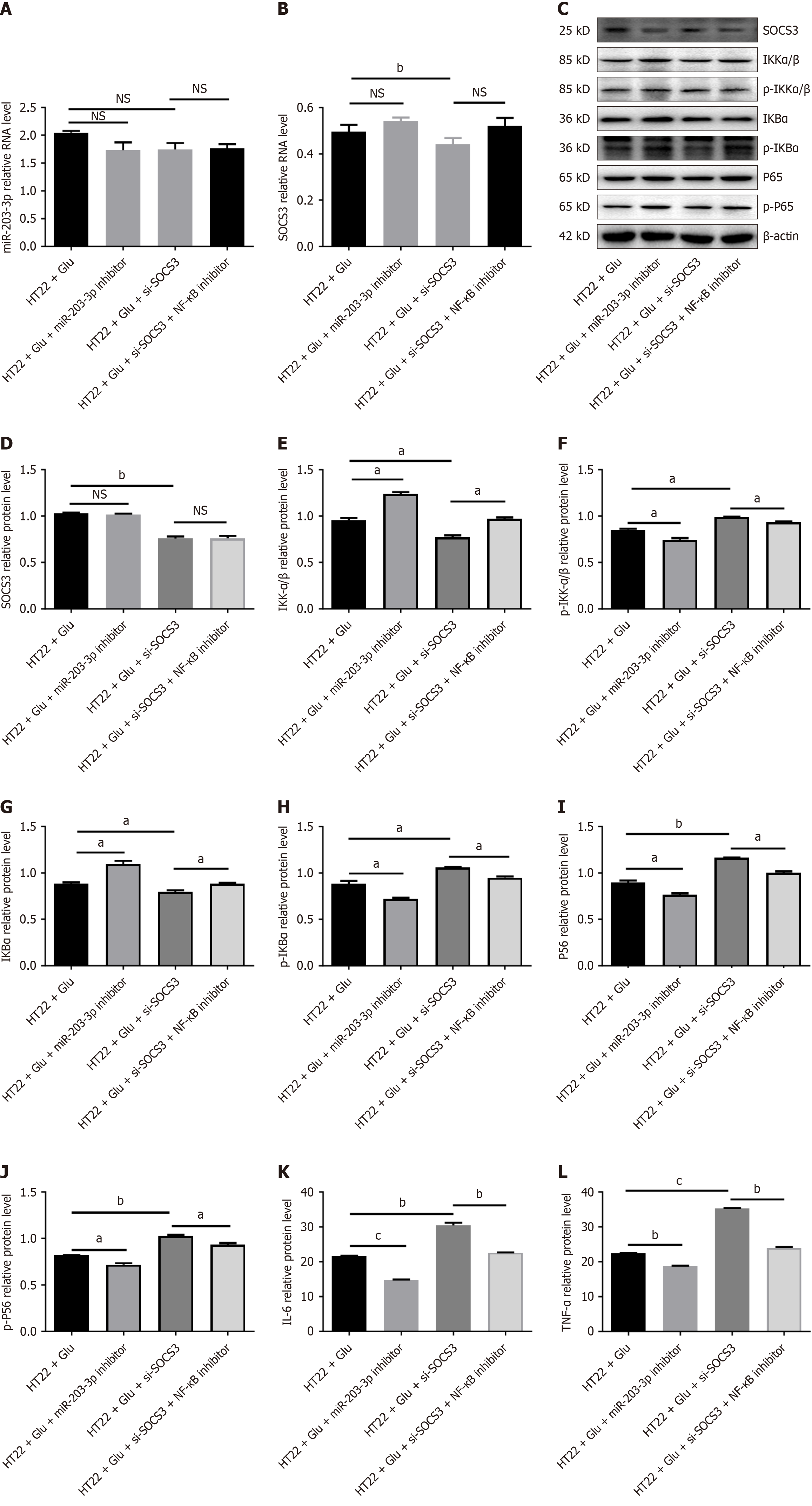
Figure 6 Inhibition of the target gene suppression of cytokine signaling 3 can activate the nuclear factor kappaB pathway and promote neuroinflammation.
A: Schematic representation of the experimental design for HT22 cells transfected with si-suppression of cytokine signaling 3 (SOCS3) or si-NC, with or without nuclear factor kappaB pathway inhibitor treatment; B: Quantitative polymerase chain reaction analysis of SOCS3 mRNA expression in HT22 cells after transfection; C-J: Western blot analysis of SOCS3 protein levels and nuclear factor kappaB pathway markers in HT22 cells under the same conditions as Figure 6C; K and L: Enzyme-linked immunosorbent assay measurements of interleukin-6 and tumor necrosis factor-α levels in the supernatant of HT22 cells treated as in Figure 6C. Data are presented as mean ± SEM of three independent experiments. aP < 0.05, bP < 0.01, cP < 0.001, NS: No significance. SOCS3: Suppression of cytokine signaling 3; NF-κB: Nuclear factor kappaB; IKKα/β: Inhibitor of kappa B kinase alpha/beta; p-IKKα/β: Phospho-inhibitor of kappa B kinase alpha/beta; IKBα: Nuclear factor kappaB inhibitor alpha; p-IKBα: Phospho-nuclear factor kappaB inhibitor alpha; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
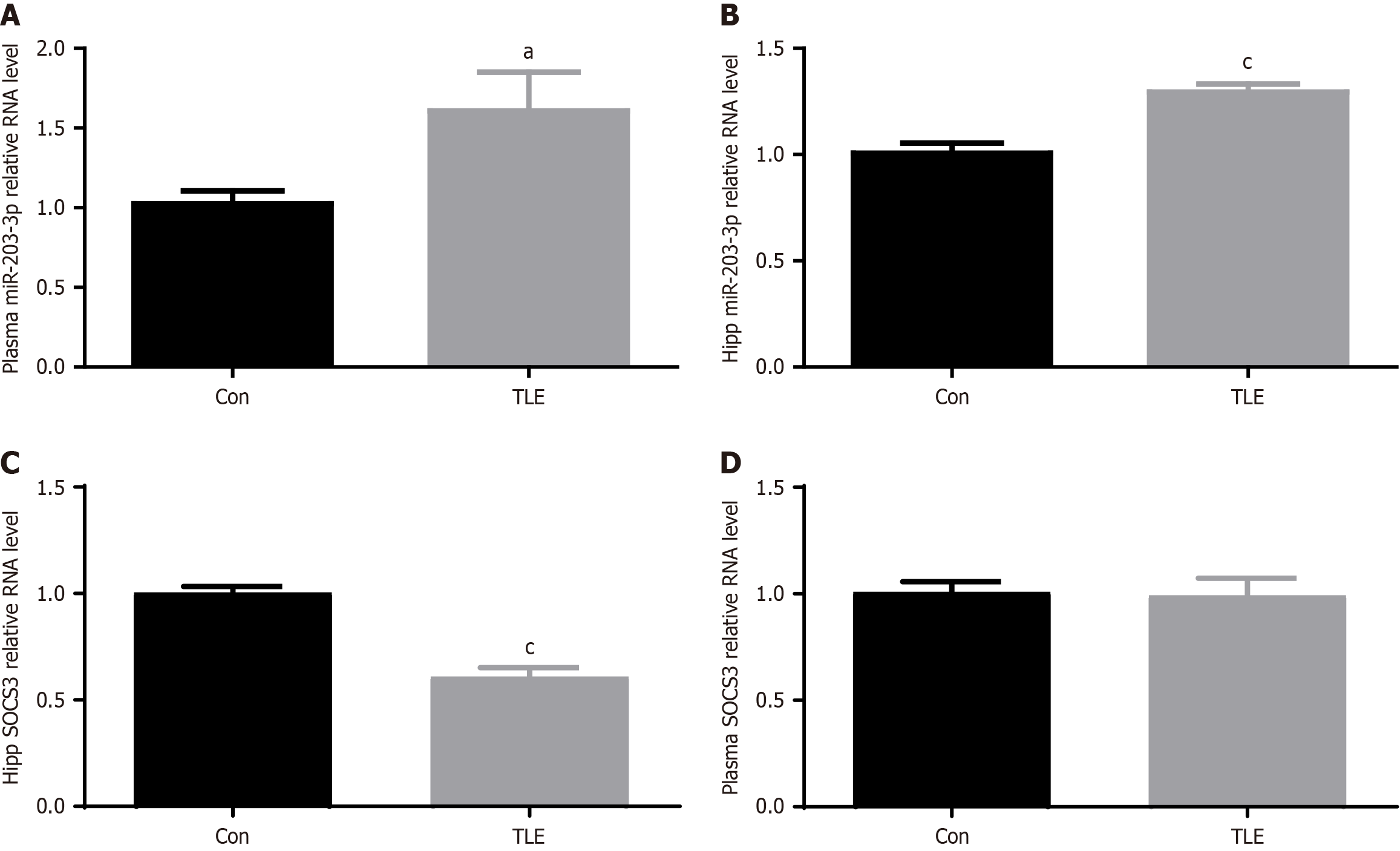
Figure 7 Differences between temporal lobe epilepsy mouse plasma and the expression of miR-203-3p and suppression of cytokine signaling 3 in the hippocampal region.
A-D: Quantitative polymerase chain reaction analysis of miR-203-3p and suppression of cytokine signaling 3 mRNA expression in the plasma and hippocampal regions of the temporal lobe epilepsy model. aP < 0.05, cP < 0.001. TLE: Temporal lobe epilepsy.
- Citation: Wang W, Yin J. Exosomal miR-203 from bone marrow stem cells targets the SOCS3/NF-κB pathway to regulate neuroinflammation in temporal lobe epilepsy. World J Stem Cells 2025; 17(2): 101395
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/101395.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.101395