Copyright
©The Author(s) 2024.
World J Stem Cells. Feb 26, 2024; 16(2): 102-113
Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.102
Published online Feb 26, 2024. doi: 10.4252/wjsc.v16.i2.102
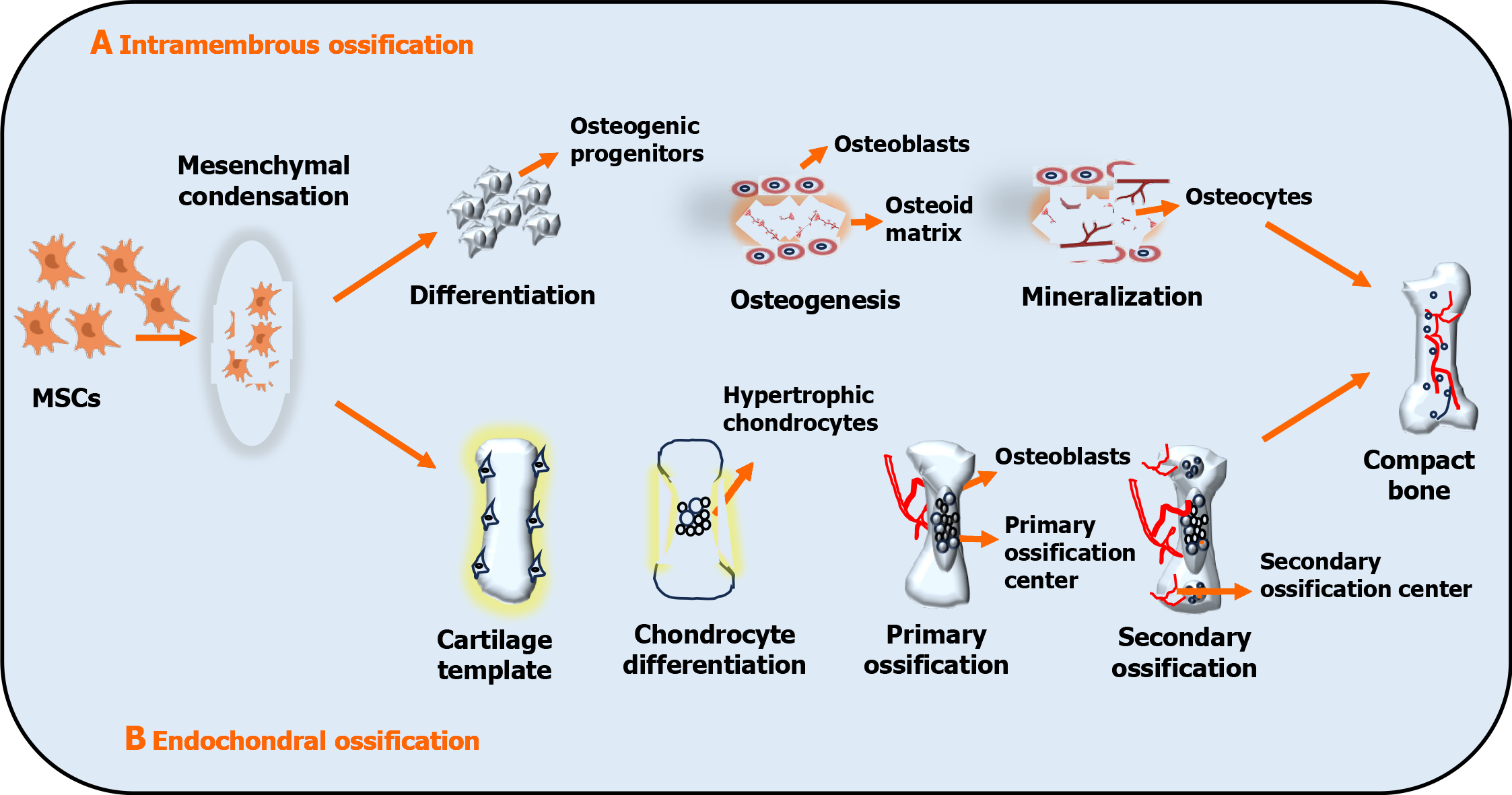
Figure 1 Schematic representation of intramembranous and endochondral ossification.
A: Undifferentiated mesenchymal cells develop into osteoprogenitor cells (osteoblasts), which lay down the osteoid matrix and mineralize to form ossification centers. Osteoblasts die due to apoptosis or become trapped in the matrix, developing into osteocytes; B: Condensed mesenchymal cells commit to become the cartilage and undergo chondrogenic differentiation. Chondrocytes at the primordium core establish a growth plate and undergo hypertrophy. Hypertrophic chondrocytes calcify and are penetrated by microvessels, resulting in primary ossification. Vessels infiltrate the epiphyses and produce secondary ossification centers in conjunction with osteoblasts and bone marrow. The growth plate aids in long bone formation. MSCs: Mesenchymal stem cells.
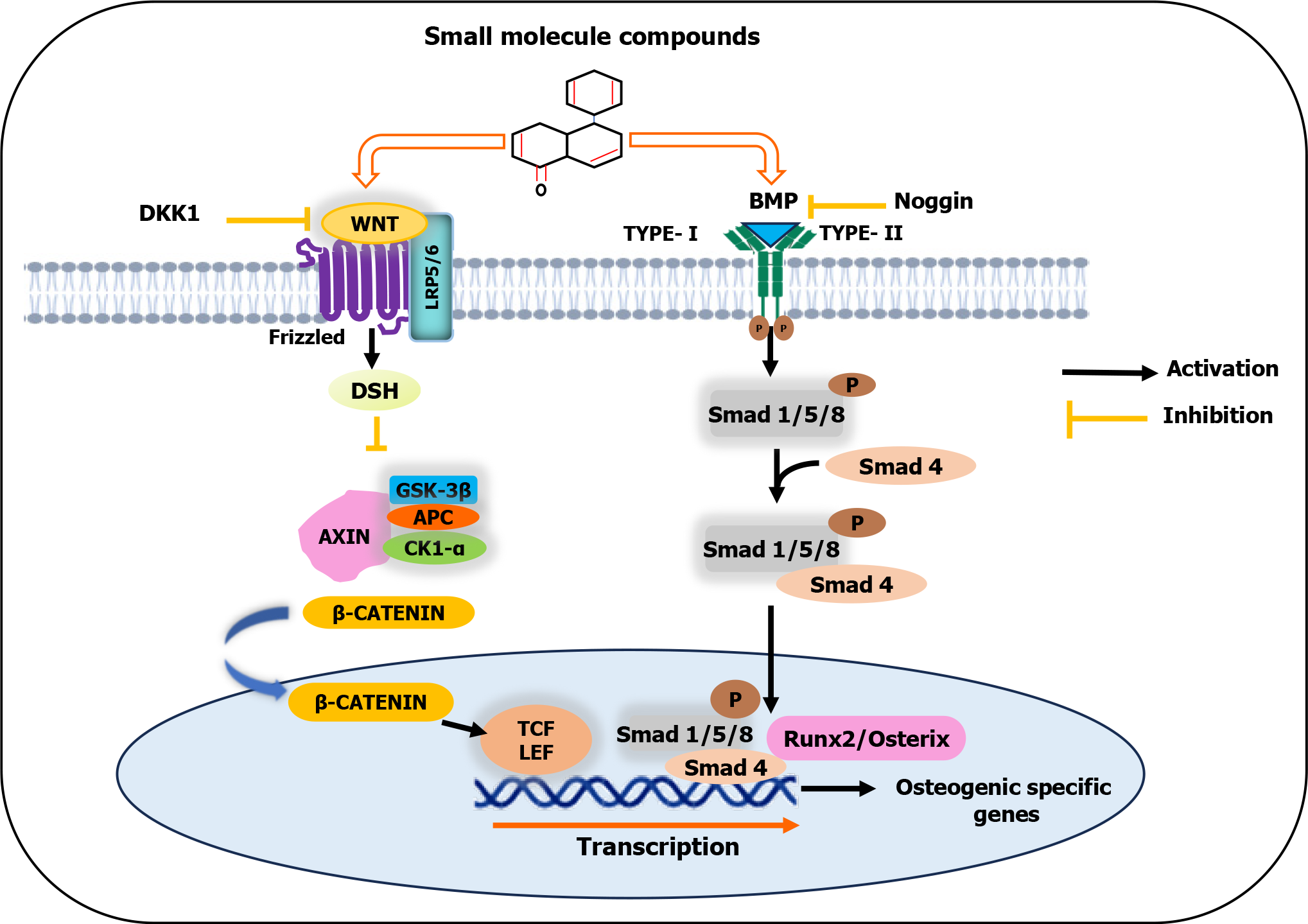
Figure 2 Schematic illustration of small molecule compounds inducing the osteogenic differentiation of mesenchymal stem cells.
Small molecule compounds stimulate the bone morphogenetic protein signaling pathway and promote β-catenin accumulation. Then, β-catenin migrates to the nucleus and forms a β-catenin-T-cell factor/lymphoid enhancer-binding factor complex to initiate the transcription of genes associated with osteogenesis. GSK-3β: Glycogen synthase kinase 3 beta; APC: Adenomatosis polyposis coli; CK1-α: Casein kinase 1α; DSH: Disheveled; TCF/LEF: T-cell factor/lymphoid enhancer-binding factor; DKK1: Dickkopf 1; LRP5/6: Lipoprotein receptor-related proteins 5 or 6; BMP: Bone morphogenetic protein.
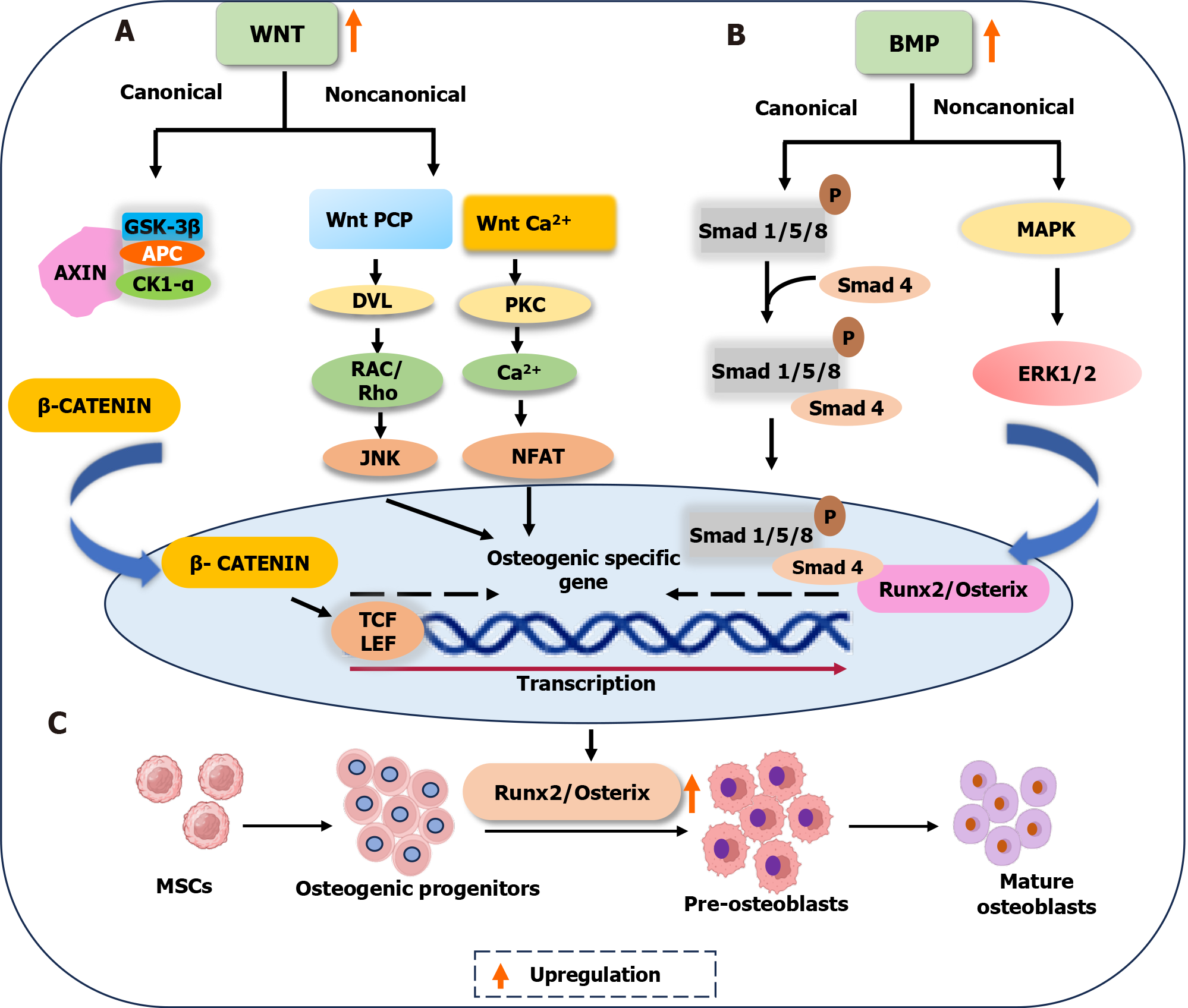
Figure 3 Schematic illustration of the crosstalk between the Wnt and bone morphogenetic protein signaling pathways in osteogenesis.
A: Binding of Wnt-specific ligand initiates the Wnt signaling pathway. In canonical pathway, activated disheveled 1 protein suppresses glycogen synthase kinase 3 and inhibits β-catenin degradation, thereby increasing the osteogenic gene expression. In non-canonical signaling, both Wnt Ca2+-dependent and Wnt planar cell polarity contribute to the upregulation of osteogenic gene expression; B: Bone morphogenetic proteins (BMPs) bind to the heterodimeric type I/II BMP transmembrane serine/threonine kinase receptors and activate the BMP signaling pathway. The canonical pathway becomes active when the BMP ligand binds to its specific receptor, inducing the phosphorylation and binding of receptor-Smads (Smad1/5/8) to the common Smad (Smad4). The translocation of this complex to the nucleus then modulates the expression of BMP target genes and osteogenic differentiation. In non-canonical pathway, activated mitogen-activated protein kinase induces extracellular signal-regulated kinase 1/2 and contributes to the upregulation of Runx2 expression; C: Upregulated Wnt and BMP signaling pathways induce the expression of Runx2/Osterix genes, followed by the expression of osteoblast differentiation genes, resulting in osteogenesis. This involves mesenchymal stem cell differentiation into osteogenic progenitors, pre-osteoblasts, and mature osteoblasts. GSK-3β: Glycogen synthase kinase 3 beta; APC: Adenomatosis polyposis coli; CK1-α: Casein kinase 1 alpha; PKC: Protein kinase C; NFAT: Nuclear factor of activated T cells; Rho: Ras homolog gene family; RAC: Ras-related C3 botulinum toxin substrate; JNK: Jun N-terminal kinase; DVL: Disheveled; TCF/LEF: T-cell factor/lymphoid enhancer-binding factor; MAPK: Mitogen-activated protein kinase; ERK1/2: Extracellular signal-regulated kinase 1/2; MSCs: Mesenchymal stem cells; BMP: Bone morphogenetic protein.
- Citation: Arya PN, Saranya I, Selvamurugan N. Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation. World J Stem Cells 2024; 16(2): 102-113
- URL: https://www.wjgnet.com/1948-0210/full/v16/i2/102.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i2.102