Copyright
©The Author(s) 2024.
World J Stem Cells. Nov 26, 2024; 16(11): 956-973
Published online Nov 26, 2024. doi: 10.4252/wjsc.v16.i11.956
Published online Nov 26, 2024. doi: 10.4252/wjsc.v16.i11.956
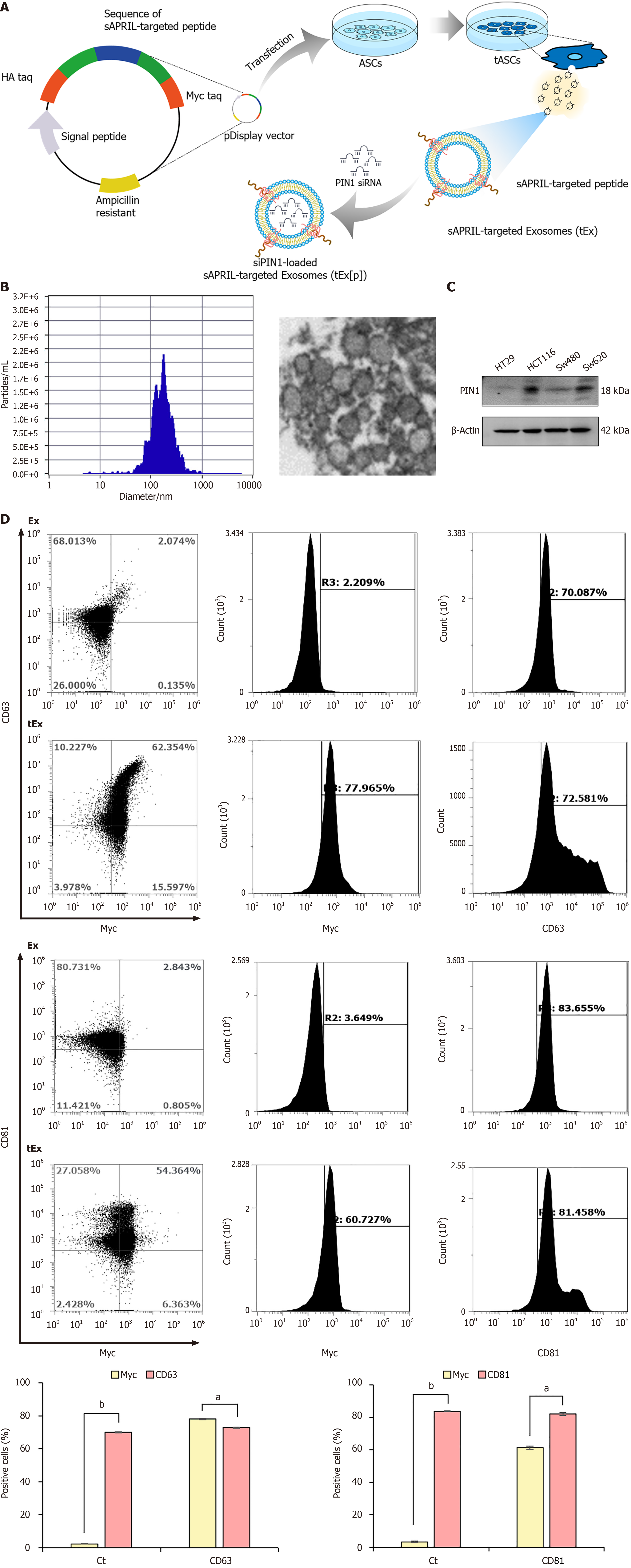
Figure 1 Generation of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome.
A: Generation of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA (siPIN1)-loaded soluble a proliferation-inducing ligand (sAPRIL)-targeted exosome (tEx[p]). Briefly, the DNA sequence of the sAPRIL-targeting peptide was integrated into a pDisplay vector and used to transfect the adipose-derived stem cell (ASC), leading to the expression of the sAPRIL peptide on the cell membranes and the secretion of tEx. Subsequently, sIPIN1 was incapsulated into tEx using Exofect kit; B: Nanoparticle analysis of sAPRIL-targeted Ex. Zetaview analysis revealed that tEx had an average size of 187.9 ± 105.3 nm, aligning with the typical size range of exosomes (Ex), as corroborated by the transmission electron microscopy images; C: Western blot analysis showing PIN1 expression in various colon cancer cells. The increased expression of PIN1 was noted in HCT116 and SW620 colon cancer cells; D: Flow cytometric analysis of tEx. The tEx showed expression of markers CD63 and CD81 similar to control levels, while specifically expressing the myc marker (tEx marker) at a rate of 61.3% to 78.1%. Values are presented as mean ± standard deviation of three independent experiments. aP < 0.05, bP < 0.01. PIN1: Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1.
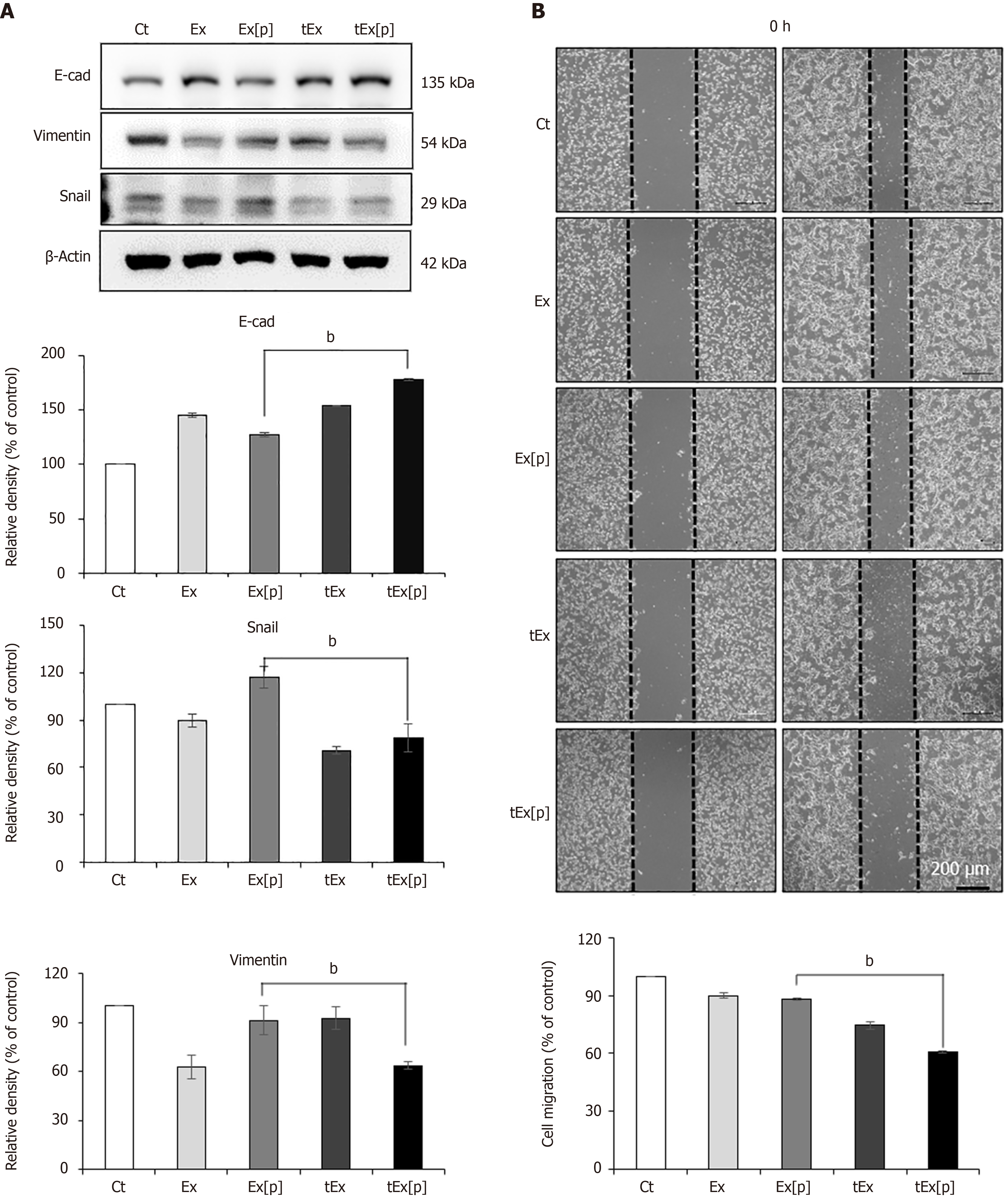
Figure 2 In vitro efficacy of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome in HCT116 colon cancer cells.
A: Western blot analysis demonstrating epithelial-mesenchymal transition-inhibiting ability of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome (tEx[p]). The tEx[p] group exhibited a significant increase in the epithelial marker E-cadherin (P < 0.05) and a decrease in mesenchymal markers Snail and Vimentin in HCT colon cancer cells; B: Wound healing assay demonstrating the inhibition of cell migration by tEx[p]. The tEx[p] group showed a marked reduction in cell migration, indicating the most effective inhibition of cell movement among all groups (P < 0.05). Relative densities of individual markers had been quantified using ImageJ software and then were normalized to that of β-actin in each group. bP < 0.01. Ex: Exosome.
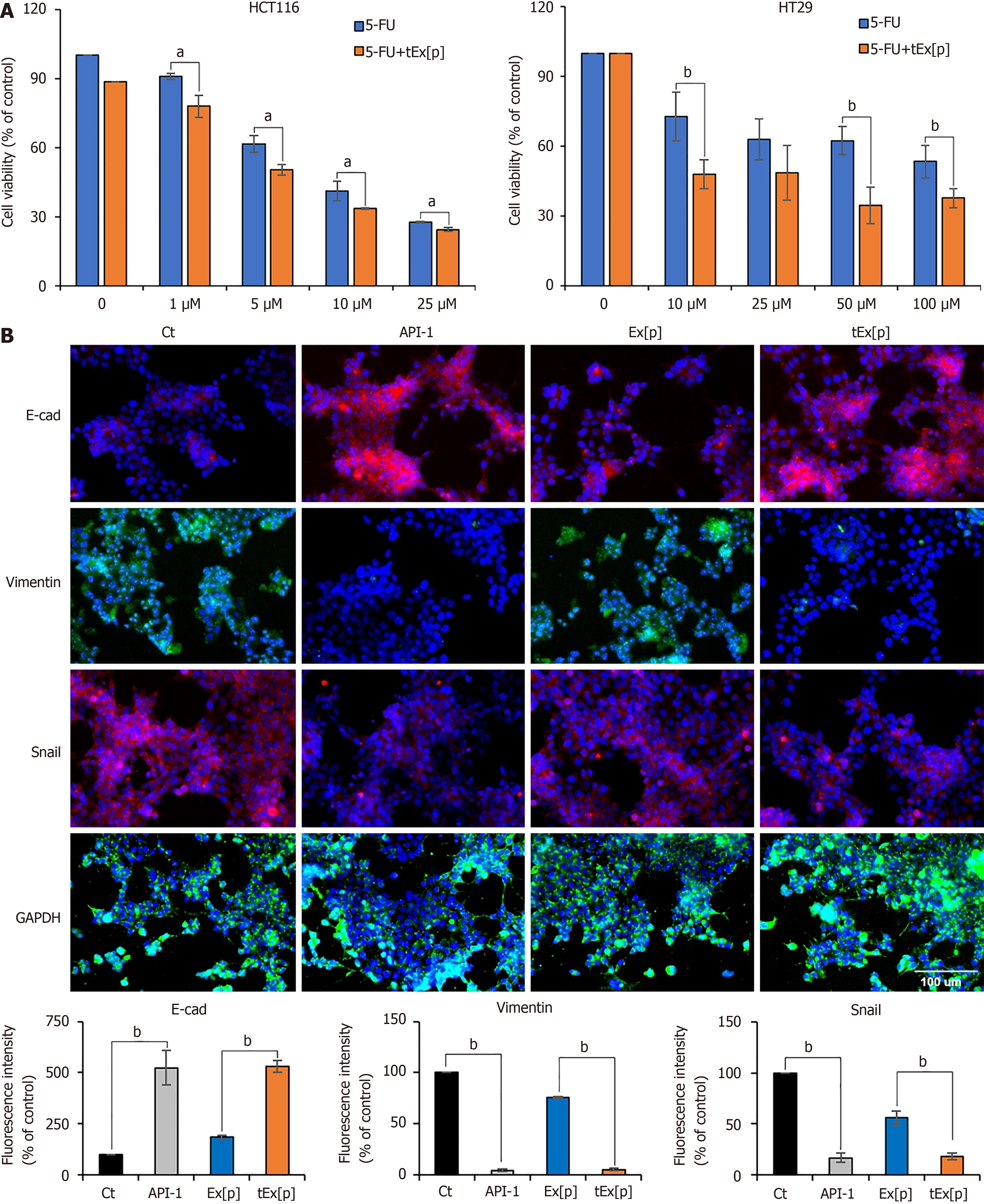
Figure 3 Enhancing chemosensitivity and inhibiting epithelial-mesenchymal transition in colon cancer cell lines using small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded exosomes.
A: Cell viability assay under increasing concentrations of 5-fluorouridine (5-FU) alone and in combination with small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA (siPIN1)-loaded soluble a proliferation-inducing ligand-targeted exosome (tEx[p]) in HCT116 (left) and HT29 (right) colon cancer cell lines. This figure illustrates the cell viability expressed as a percentage relative to untreated controls in two distinct colon cancer cell lines subjected to escalating doses of 5-FU, both alone and in conjunction with tEx[p]. The data highlight that the concomitant use of tEx[p] with 5-FU could enhance the chemotherapeutic response by further diminishing cancer cell survival; B: Immunofluorescence of epithelial-mesenchymal transition (EMT) markers upon treatment of API-1 (PIN inhibitor) and siPIN encapsulated exosomes (Ex). Immunofluorescence of EMT markers was performed for the determination of the effects of API-1, Ex(siPIN1), and tEx(siPIN1) on EMT in HCT116 cells. Treatment of API-1 (20 μM) and tEx(siPIN1; 100 pmol) effectively inhibited EMT, as demonstrated by the highest increase in E-cadherin expression and the lowest decrease in Snail and Vimentin expression among all treatment groups. Percentages of immunoreactive areas were measured using NIH ImageJ and expressed as relative values to those in control cells. Values are presented as mean ± standard deviation (SD) of three independent experiments. Values are presented as mean ± SD of three independent experiments. aP < 0.05, bP < 0.01.
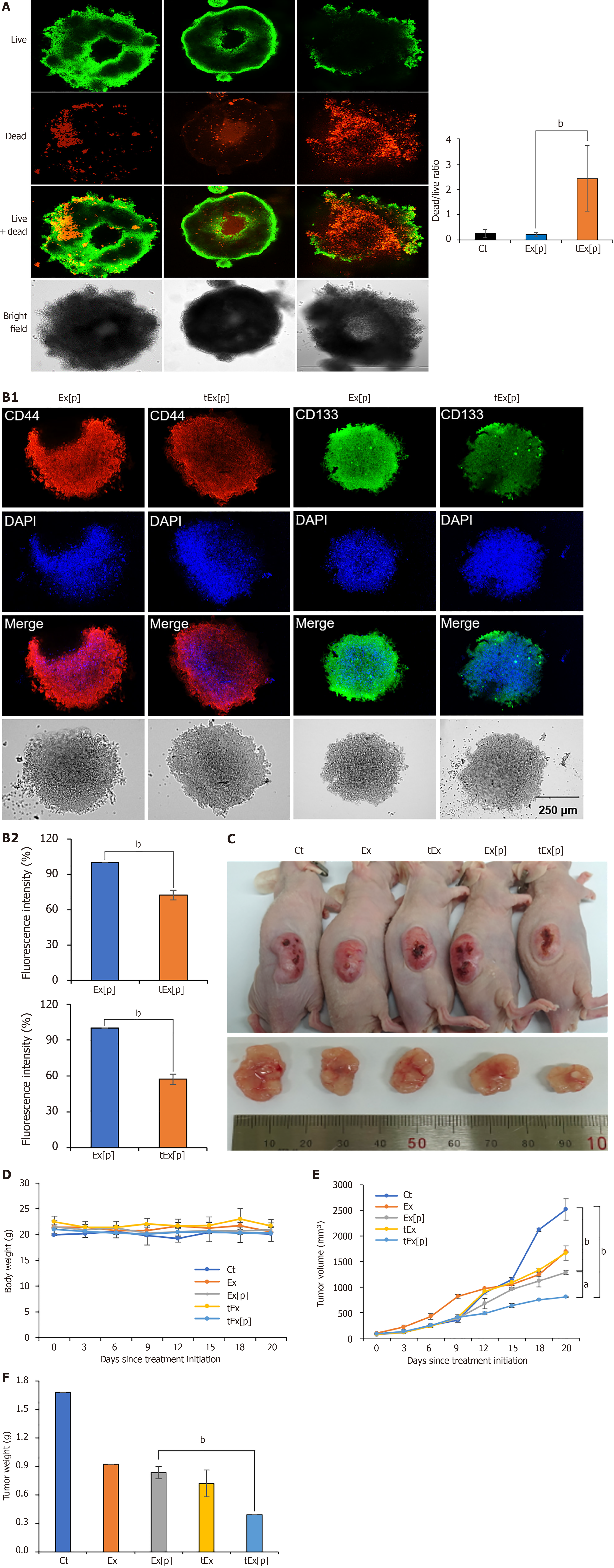
Figure 4 Spheroid-based and in vivo assessment of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome efficacy in HCT116 models.
A: Effects of exosomes (Ex) loaded with siPIN1 (Ex[p]) and small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome (tEx[p]) on cell viability in HCT116-derived spheroids. Fluorescence microscopy images illustrate cell viability after treatments. Cells were stained using a LIVE/DEAD staining kit to distinguish live cells (green) from dead cells (red) (left panel). Representative images of spheroids treated with negative control, Ex[p], and tEx[p] (right panel). Quantification of the dead/live ratio, showing a significant increase in cell death in the tEx[p] treated spheroids compared to Ex[p] treated ones. This increase suggests that tEx[p] could also affect the viability of cancer stem cells within the spheroids, indicating its potential efficacy against tumor resilience. Error bars denote standard deviation based on three independent experiments; B: Immunofluorescence analysis of cancer stem cell markers CD44 and CD133 in spheroids treated with Ex[p] and tEx[p] for 24 hours. The expression of CD44 (red) and CD133 (green) is reduced in tEx[p] treated spheroids compared to Ex[p] treated spheroids, indicating a decrease in cancer stem cell populations. Nuclei are counterstained with DAPI (blue). Images are representative of three independent experiments; C: Xenograft appearance and size comparison in each group; D: Comparision of body weight measurements, demonstrating no significant weight differences among the groups, suggesting minimal systemic toxicity; E: Measurements of tumor volumes. The tEx[p] group demonstrated the smallest tumor volumes in the comparison of tumor sizes; F: Measurements of tumor volumes on day 20 post-treatment. The tEx[p] group exhibited significantly reduced tumor weights compared to other groups in the tumor weight comparison. aP < 0.05, bP < 0.01.
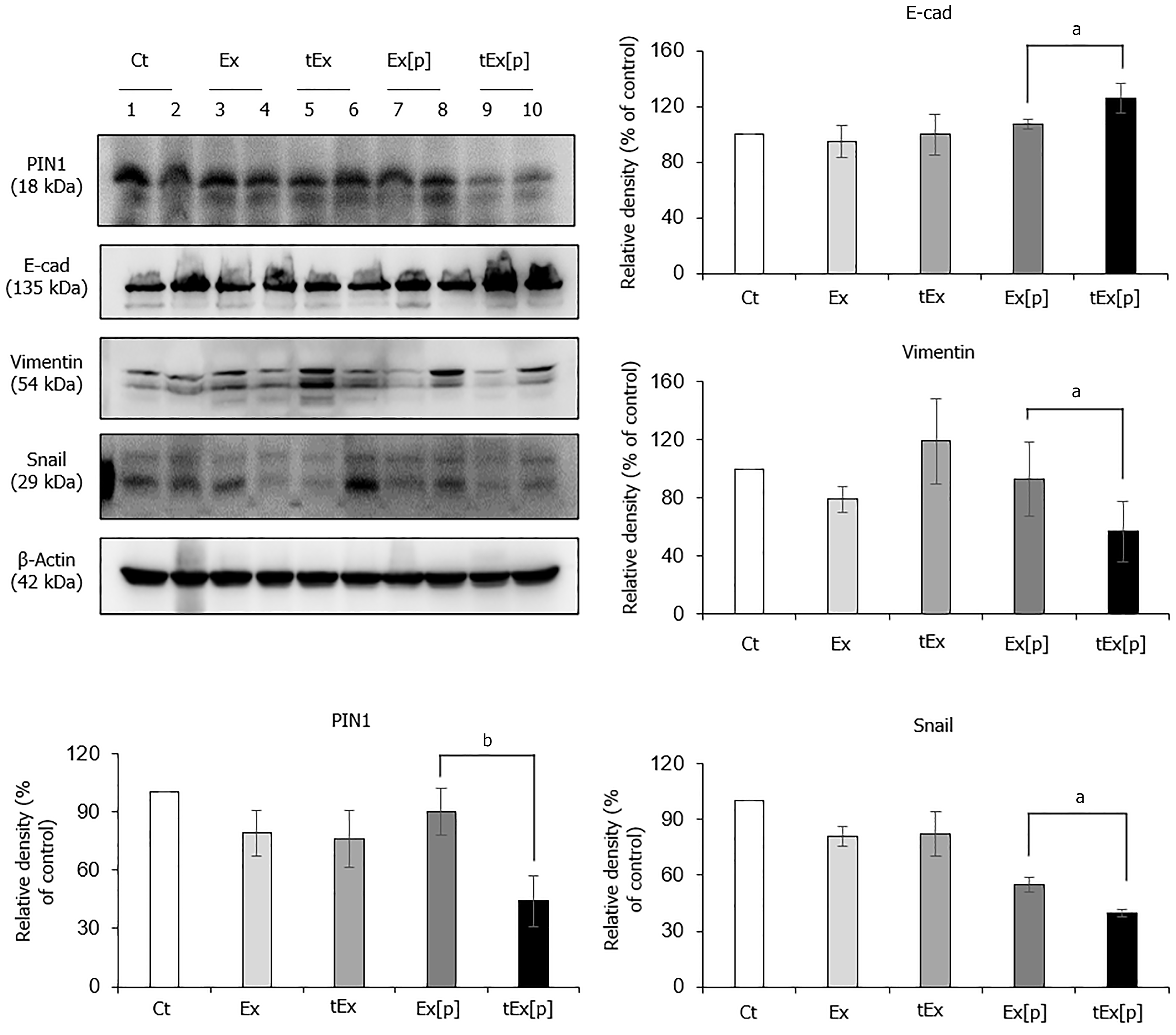
Figure 5 Anticancer effects of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome in excised tumor tissues.
Western blot analysis of excised tumor tissues revealed that the small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome (tEx[p]) group showed the lowest peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (P1N1) expression levels, coupled with the highest expression of the epithelial marker E-cadherin, and the lowest levels of mesenchymal markers Vimentin and Snail (P < 0.05), suggesting that tEx[p] effectively inhibits epithelial-mesenchymal transition in tumor cells. Relative densities of individual markers had been quantified using Image J software and then were normalized to that of β-actin in each group. Values are presented as mean ± standard deviation of three independent experiments. aP < 0.05, bP < 0.01. Ex: Exosome.
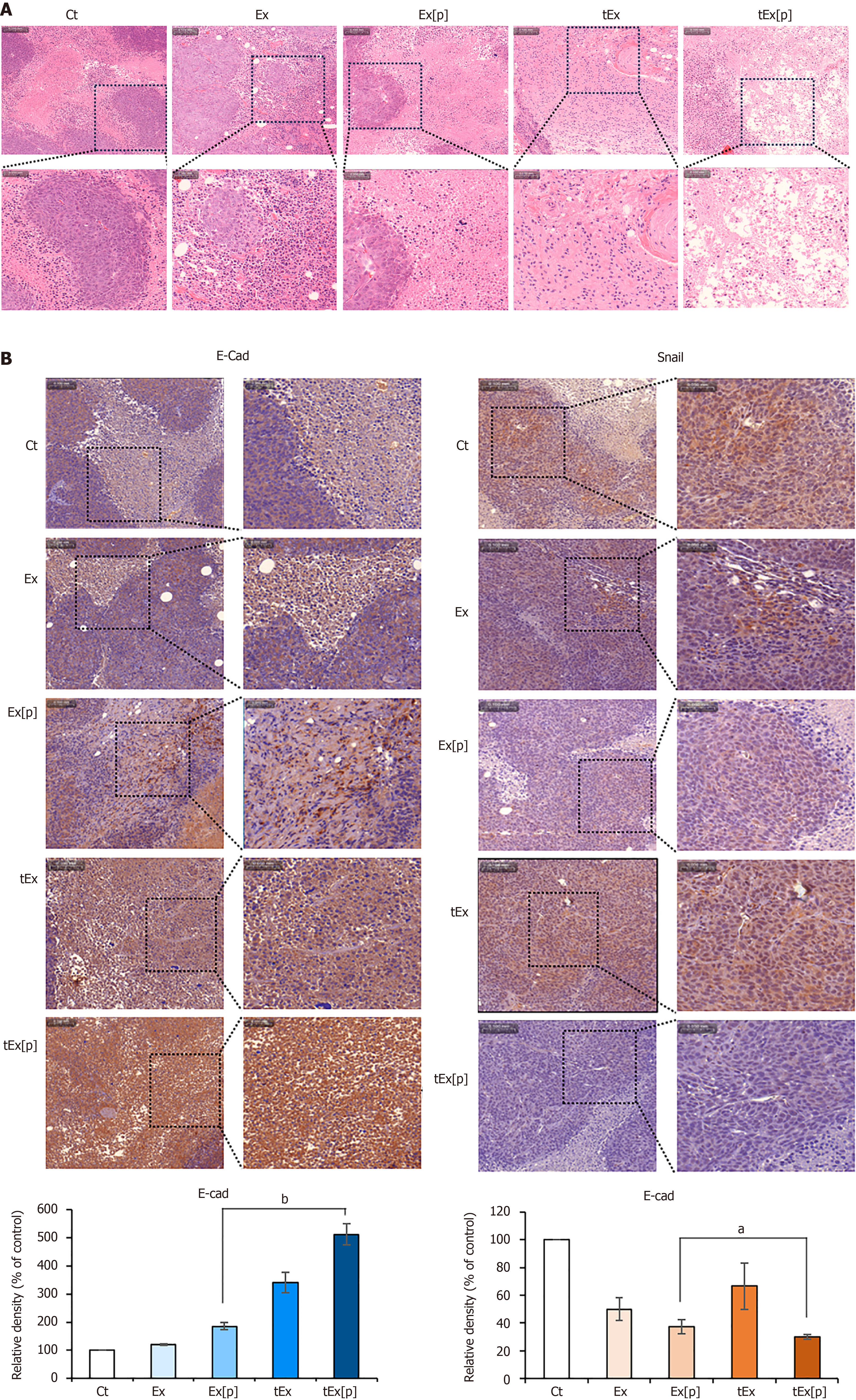
Figure 6 Histological analysis of excised tumor tissues in mouse colorectal cancer xenograft model.
A: Hematoxylin and eosin staining, showing that the small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosome (tEx[p]) group exhibited a significantly reduced tumor cell density, indicating a pronounced effect on tumor growth suppression; B: Immunohistochemical analysis of epithelial-mesenchymal transition-related markers. The tEx[p] group showed increased immunoreactivity of the epithelial marker E-cadherin (left), while decreasing immunoreactivity of the mesenchymal marker Snail (right), underscoring its efficacy in inhibiting the epithelial-mesenchymal transition process in the colon cancer tissues. Values are presented as mean ± standard deviation of three independent experiments. Percentages of immunoreactive areas were measured using NIH ImageJ and expressed as relative values to those in control tissues. aP < 0.05, bP < 0.01. Ex: Exosome.
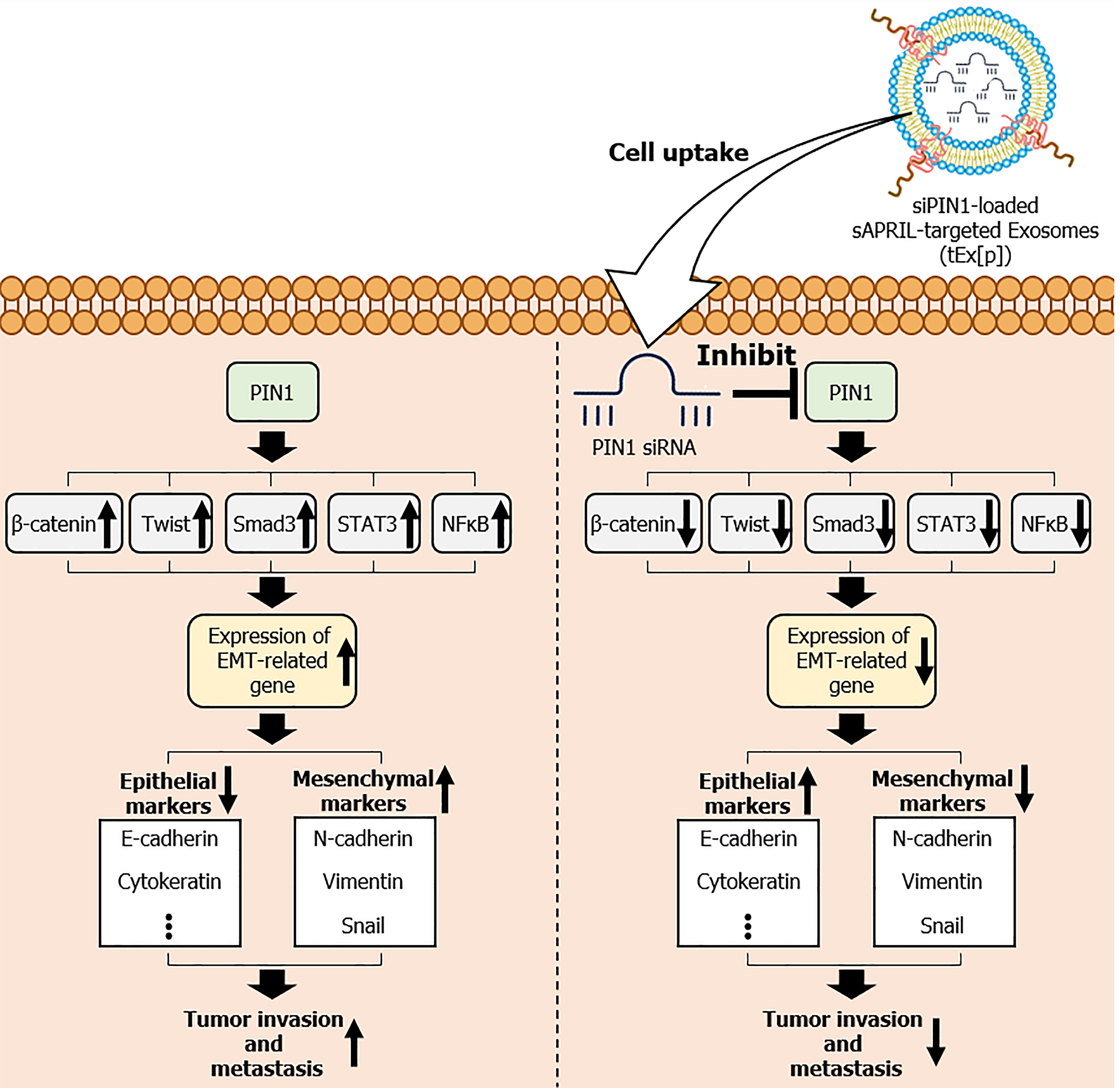
Figure 7 Mechanism of small interfering peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 RNA-loaded soluble a proliferation-inducing ligand-targeted exosomes in inhibiting epithelial-mesenchymal transition and tumor progression.
Overexpression of peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1) leads to the downregulation of epithelial markers, such as E-cadherin and cytokeratin, and the upregulation of mesenchymal markers, including N-cadherin, Vimentin, and Snail. These changes facilitate epithelial-mesenchymal transition (EMT) through the activation of key signaling pathways involving β-catenin, Twist, small mothers against decapentaplegic 3 (Smad3), signal transducer and activator of transcription 3 (STAT3), and nuclear factor-kappa B (NF-κB), resulting in increased cell migration and invasiveness. The figure also highlights the therapeutic intervention using small interfering siPIN1 RNA (siPIN1)-loaded soluble a proliferation-inducing ligand-targeted exosomes (tEx[p]), which effectively deliver siPIN1 to tumor cells, inhibiting PIN1 activity. This targeted delivery reduces EMT, thereby decreasing tumor invasiveness and exhibiting significant antitumor effects. Arrows indicate the flow and direction of these molecular interactions and the impact of tEx[p] treatment in reversing EMT progression. sAPRIL: Soluble a proliferation-inducing ligand.
- Citation: Kim HJ, Lee DS, Park JH, Hong HE, Choi HJ, Kim OH, Kim SJ. Exosome-based strategy against colon cancer using small interfering RNA-loaded vesicles targeting soluble a proliferation-inducing ligand. World J Stem Cells 2024; 16(11): 956-973
- URL: https://www.wjgnet.com/1948-0210/full/v16/i11/956.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i11.956