Copyright
©The Author(s) 2024.
World J Stem Cells. Oct 26, 2024; 16(10): 873-895
Published online Oct 26, 2024. doi: 10.4252/wjsc.v16.i10.873
Published online Oct 26, 2024. doi: 10.4252/wjsc.v16.i10.873
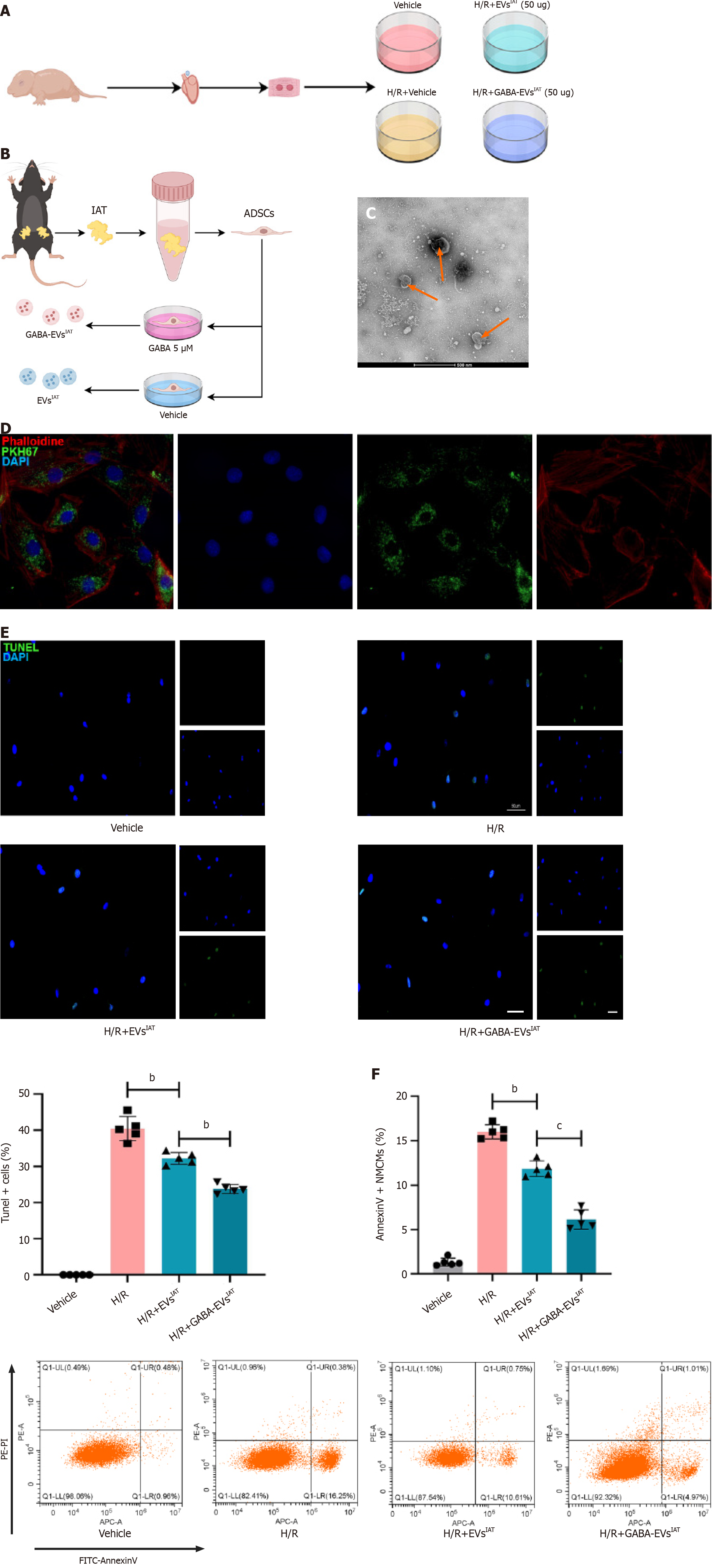
Figure 1 Gamma-aminobutyric acid-induced adipose-derived mesenchymal stem cells from inguinal adipose tissue exhibits potential in mitigating neonatal mouse cardiomyocytes injury under hypoxia and reoxygenation conditions in vitro.
A: Experiment design. The cardiomyocytes extracted from neonatal mice were categorized into four experimental groups: Vehicle, hypoxia and reoxygenation (H/R), H/R+ extracellular vesicles (EVs) derived from adipose-derived mesenchymal stem cells (ADSCs) from inguinal adipose tissue (IAT), H/R+ gamma-aminobutyric acid (GABA)-induced ADSCs from IAT; B: Experiment design. ADSCs were extracted from the IAT of mice using an enzymatic digestion method. After incubating the ADSCs in complete medium containing 5 μM GABA for 48 h, EVs were extracted; C: Representative micrographs of EVs examined by transmission electron micrographs (scale bars = 500 nm); D: Representative micrographs of PKH67 (scale bars = 200 nm); E: Representative micrographs of isolated neonatal mouse cardiomyocytes stained with terminal deoxynucleotidyl transferase dUTP nick end-labeling (n = 5; scale bars = 200 μm); F: The percentage of AnnexinV+ neonatal mouse cardiomyocytes were calculated using flow cytometry (n = 5). bP < 0.01, cP < 0.001.
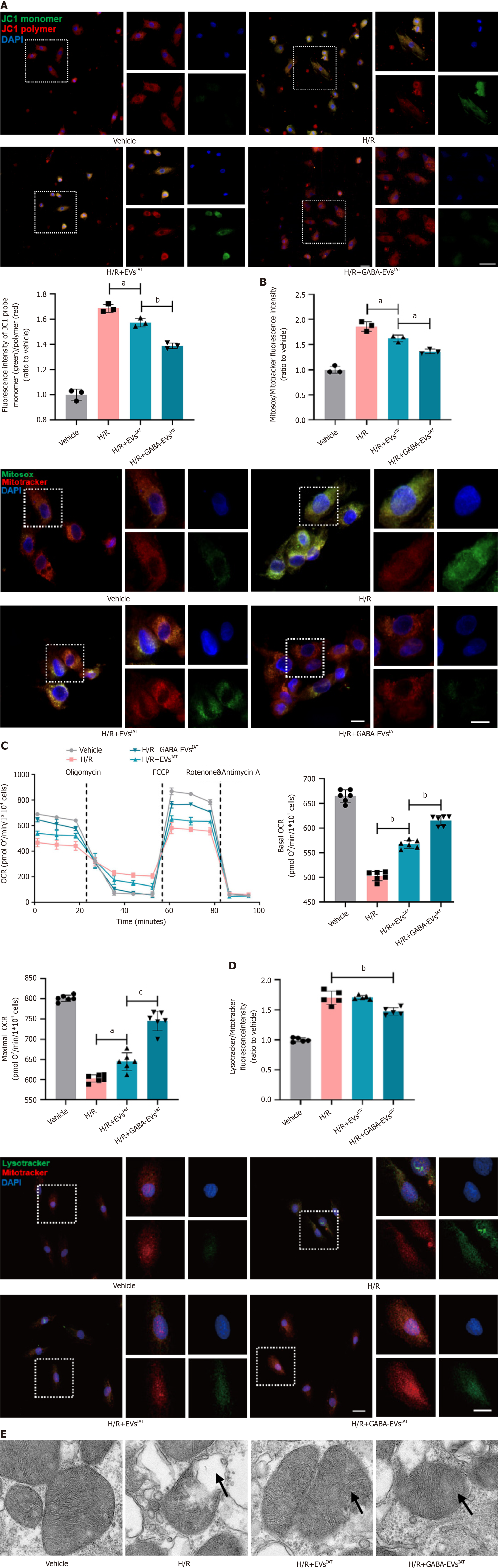
Figure 2 Gamma-aminobutyric acid-induced adipose-derived mesenchymal stem cells from inguinal adipose tissue mitigates neonatal mouse cardiomyocytes mitochondrial oxidative stress levels and enhances mitochondrial function during hypoxia and reoxygenation in vitro.
A: Representative micrographs of isolated neonatal mouse cardiomyocytes (NMCMs) stained with JC1 probe (n = 3; scale bars = 200 μm); B: Representative micrographs of isolated NMCMs stained with mitosox probe (n = 3; scale bars = 200 μm); C: Real-time oxygen consumption rates (OCRs) and calculated basal and maximal respiration rates in NMCMs (n = 6); D: Representative micrographs of isolated NMCMs stained with mitotracker and lysotracker (n = 5; scale bars = 200 μm); E: Representative micrographs of extracellular vesicles (EVs) examined by transmission electron micrographs (scale bars = 500 nm). aP < 0.05, bP < 0.01, cP < 0.001. GABA: Gamma-aminobutyric acid; H/R: Hypoxia and reoxygenation; IAT: Inguinal adipose tissue.
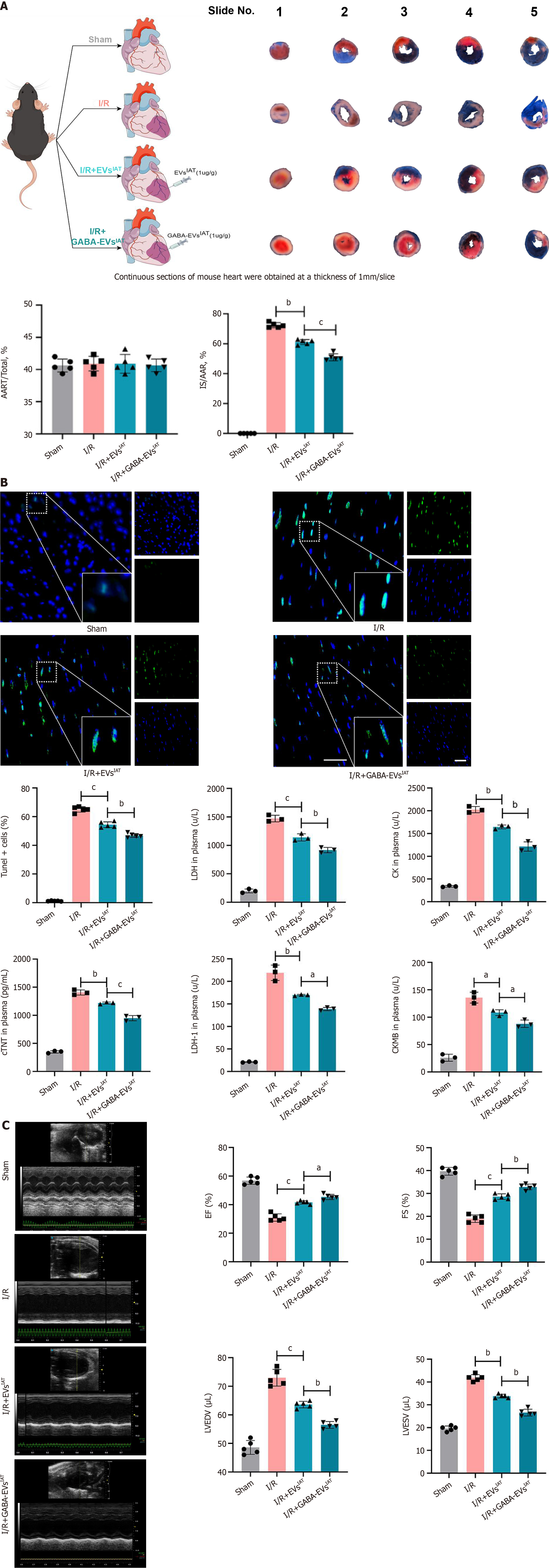
Figure 3 Gamma-aminobutyric acid-induced adipose-derived mesenchymal stem cells from inguinal adipose tissue exhibits potential in mitigating myocardial injury and improve cardiac function under myocardial ischemia-reperfusion injury conditions in vivo.
A: Representative graphs of Evans blue/triphenyltetrazolium chloride stain (n = 5); B: Representative micrographs of myocardial tissue section from the infarcted area stained with terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) (n = 5) and serum cardiac markers degree of mice (n = 3); C: Representative M-mode images of transthoracic echocardiography, and quantification of left ventricular ejection fraction, left ventricular fraction shortening, left ventricular end diastolic volume and left ventricular end systolic volume (n = 5). aP < 0.05, bP < 0.01, cP < 0.001. AAR: Area at risk; CK: Creatine kinase; cTNT: Cardiac troponin T; CKMB: Creatine kinase-MB; EF: Ejection fraction; EVs: Extracellular vesicles; FS: Fraction shortening; GABA: Gamma-aminobutyric acid; IAT: Inguinal adipose tissue; I/R: Ischemia/reperfusion; IS: Infarct size; LDH: Lactate dehydrogenase; LVEDV: Left ventricular end diastolic volume; LVESA: Left ventricular end systolic volume.
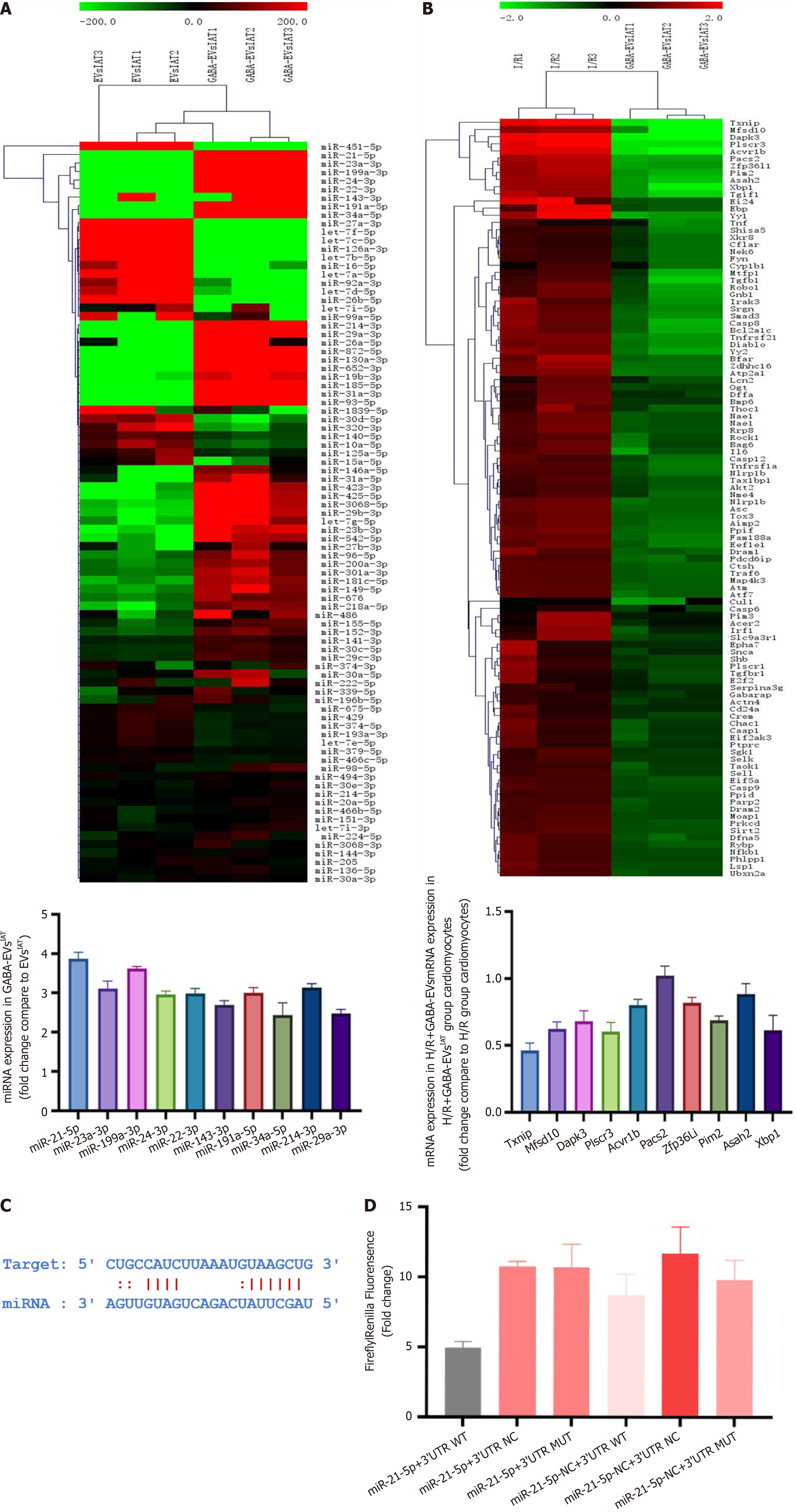
Figure 4 MicroRNA-21-5p derived from gamma-aminobutyric acid-induced adipose-derived mesenchymal stem cells from inguinal adipose tissue regulate thioredoxin-interacting protein expression in mRNA degree.
A: MicroRNA (miRNA) sequencing results of gamma-aminobutyric acid (GABA)-induced adipose-derived mesenchymal stem cells (ADSCs) from inguinal adipose tissue (IAT) vs extracellular vesicles (EVs)IAT; B: mRNA sequencing results of neonatal mouse cardiomyocytes exposed to GABA-induced ADSCs from IAT; C: Binding sites predicted for the 3’ untranslated region (UTR) regions of thioredoxin-interacting protein and miR-21-5p; D: Validation of the binding between thioredoxin-interacting protein and miR-21-5p using a dual luciferase reporter gene assay. H/R: Hypoxia and reoxygenation; miRNA: MicroRNA; MUT: Mutated; NC: Negative control; WT: Wild-type.
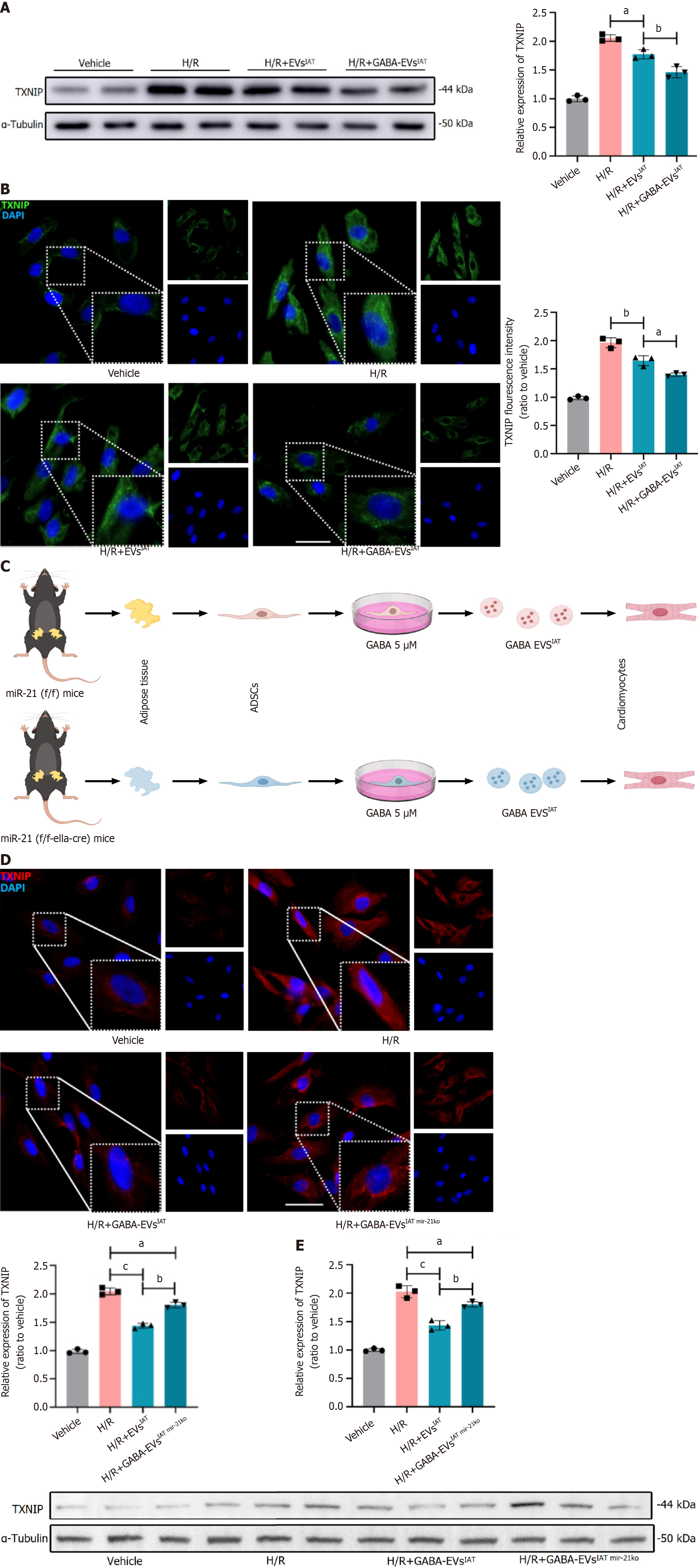
Figure 5 MicroR-21-5p derived from gamma-aminobutyric acid-induced adipose-derived mesenchymal stem cells from inguinal adipose tissue regulate thioredoxin-interacting protein expression in protein degree.
A: Representative immunoblots and quantitative analysis of thioredoxin-interacting protein (TXNIP) at the protein level in neonatal mouse cardiomyocytes (NMCMs) (n = 3); B: Representative micrographs of NMCMs stained with TXNIP antibody (n = 3; scale bars = 200 μm); C: Experiment design. Adipose-derived mesenchymal stem cells (ADSCs) were isolated from both miR-21 KO and miR-21f/f mice inguinal adipose tissue (IAT), and extracellular vesicles (EVs) were extracted respectively; D: Representative micrographs of NMCMs stained with TXNIP antibody (n = 3; scale bars = 200 μm); E: Representative immunoblots and quantitative analysis of TXNIP at the protein level in NMCMs (n = 3). aP < 0.05, bP < 0.01, cP < 0.001. GABA: Gamma-aminobutyric acid; H/R: Hypoxia and reoxygenation.
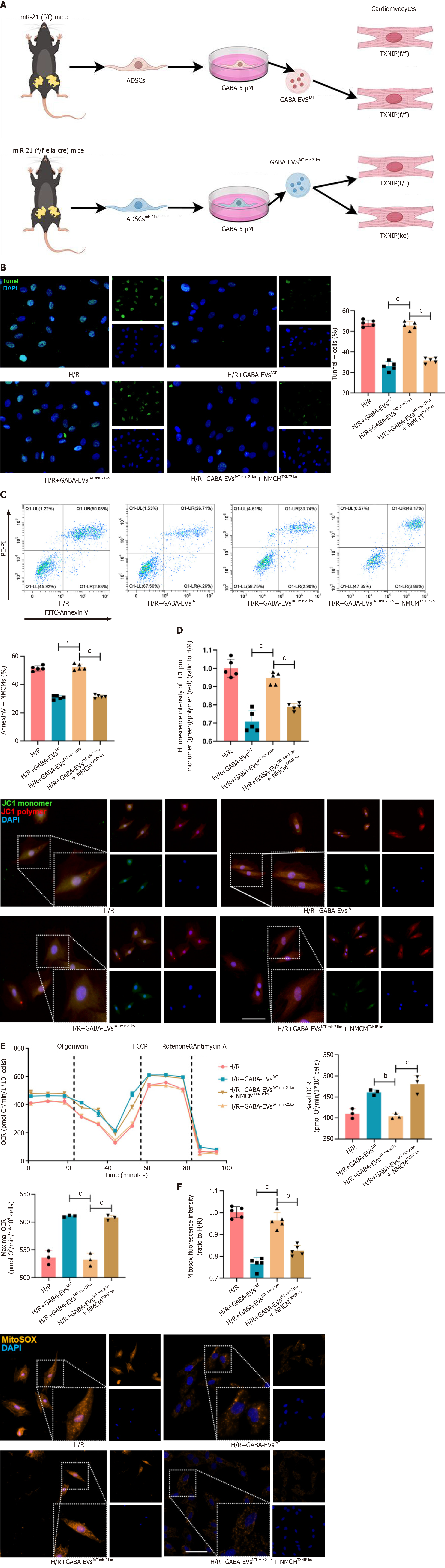
Figure 6 Extracellular vesicles derived from adipose-derived mesenchymal stem cells from inguinal adipose tissue-associated microRNA-21-5p alleviates hypoxia and reoxygenation-mediated myocardial injury and mitochondrial dysfunctions by downregulating thioredoxin-interacting protein.
A: Experiment design. Adipose-derived mesenchymal stem cells (ADSCs) were isolated from both microRNA-21 (miR-21) knockout (KO) and miR-21f/f mouse inguinal adipose tissue (IAT), and extracellular vesicles (EVs) were extracted respectively. Neonatal mouse cardiomyocytes (NMCMs) and NMCMsTXNIP KO were respectively treated with gamma-aminobutyric acid (GABA)-induced ADSCs from IAT (GABA-EVsIAT) and GABA-EVsIAT miR 21KO; B: Representative micrographs of NMCMs stained with thioredoxin-interacting protein (TXNIP) antibody (n = 5; scale bars = 200 μm); C: The percentage of AnnexinV+ NMCMs was calculated using flow cytometry (n = 5); D: Representative micrographs of isolated NMCMs stained with JC1 probe (n = 5; scale bars = 200 μm); E: Real-time oxygen consumption rates (OCRs) and calculated basal and maximal respiration rates in NMCMs (n = 3); F: Representative micrographs of isolated NMCMs stained with mitosox probe (n = 5; scale bars = 200 μm). bP < 0.01, cP < 0.001, dP < 0.0001. H/R: Hypoxia and reoxygenation; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end-labeling.
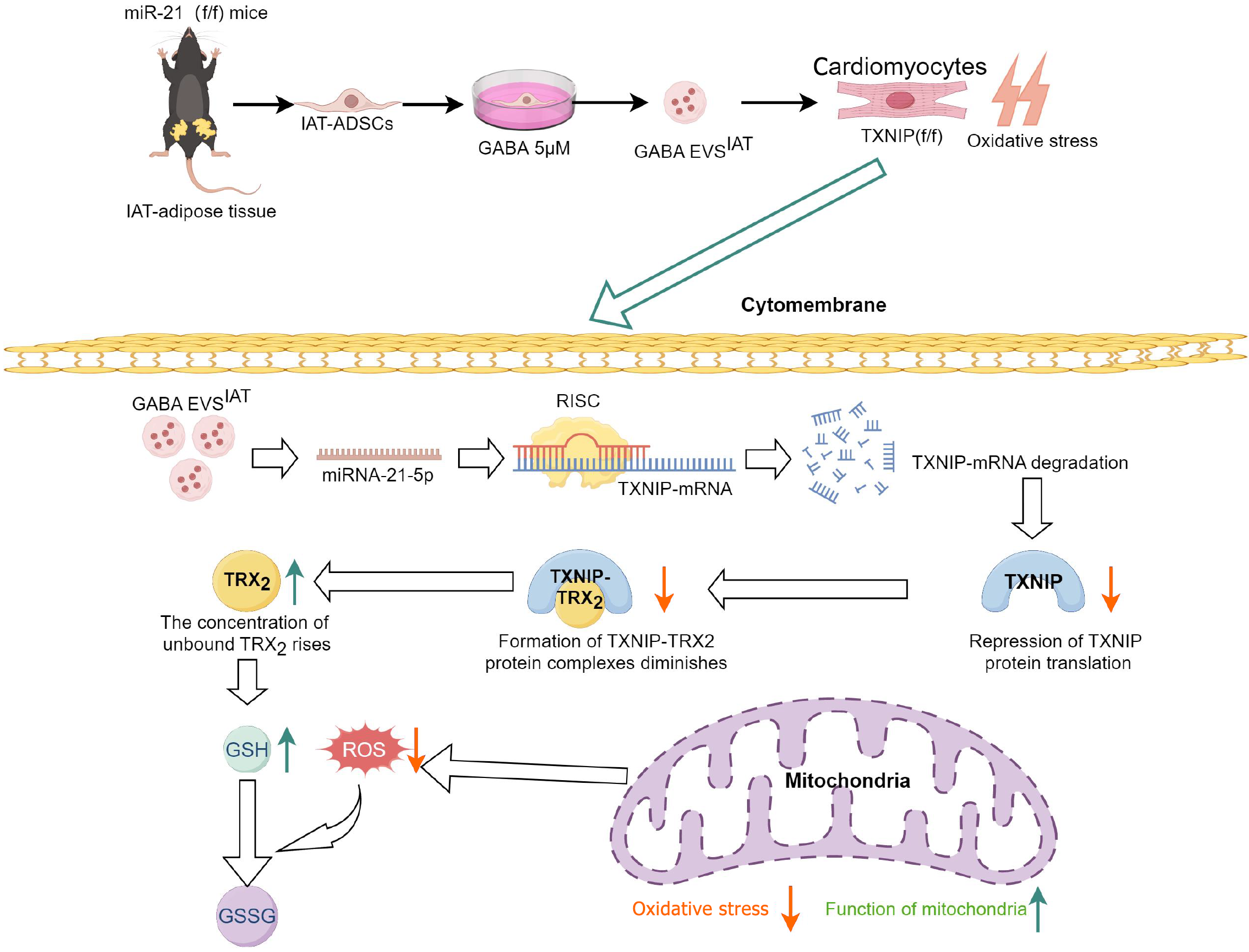
Figure 7 Graphical abstract.
Adipose-derived mesenchymal stem cells (ADSCs) derived from subcutaneous adipose tissue, under the influence of gamma-aminobutyric acid (GABA), secrete extracellular vesicles (EVs) abundant in microRNA-21-5p. These EVs possess the ability to impede intracellular thioredoxin-interacting protein (TXNIP) expression in cardiomyocytes, thereby modulating the formation of TXNIP-thioredoxin 2 complex and facilitating thioredoxin 2 involvement in regulating mitochondrial oxidative stress. Consequently, this diminishes the level of mitochondrial oxidative stress in cardiomyocytes and confers protective effects on cardiomyocytes under pathological conditions such as myocardial ischemia-reperfusion injury. GSH: Glutathione; IAT: Inguinal adipose tissue; ROS: Reactive oxygen species; TRX2: Thioredoxin 2.
- Citation: Wang FD, Ding Y, Zhou JH, Zhou E, Zhang TT, Fan YQ, He Q, Zhang ZQ, Mao CY, Zhang JF, Zhou J. Gamma-aminobutyric acid enhances miR-21-5p loading into adipose-derived stem cell extracellular vesicles to alleviate myocardial ischemia-reperfusion injury via TXNIP regulation. World J Stem Cells 2024; 16(10): 873-895
- URL: https://www.wjgnet.com/1948-0210/full/v16/i10/873.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i10.873