Copyright
©The Author(s) 2023.
World J Stem Cells. Jun 26, 2023; 15(6): 530-547
Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.530
Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.530
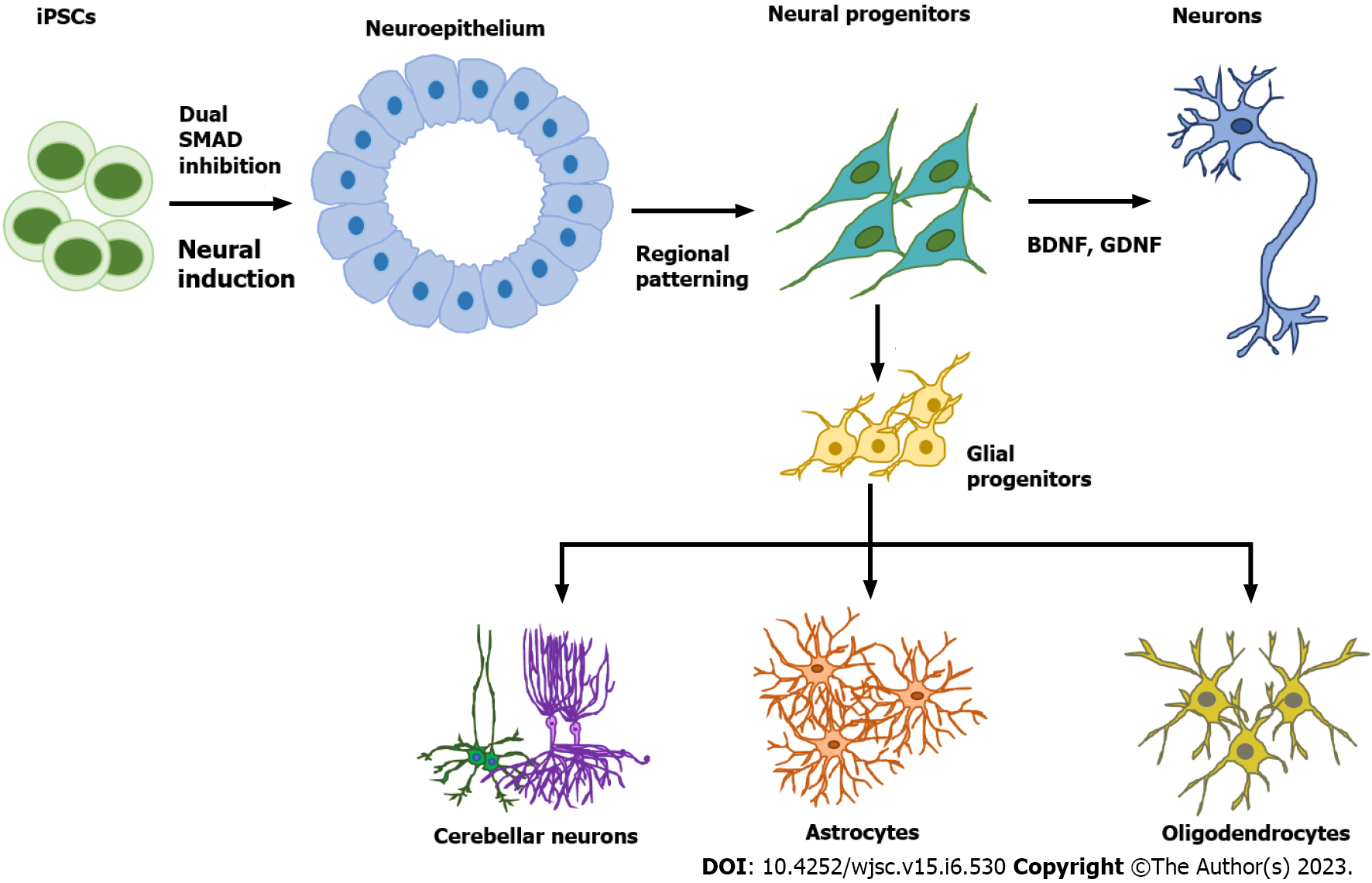
Figure 1 Neural cell subtype differentiation from human pluripotent stem cells.
The first step of neural cell differentiation is neural induction to generate neuroepithelial cells, usually by the dual SMAD inhibition method. Specific neural progenitors can be generated by tuning different signaling pathways such as Sonic Hedgehog, Wingless/integrated, retinoic acid, and bone morphogenetic protein. Neural progenitors can then be directed to become mature neurons through induction with neurotrophic factors such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor or derived into glial progenitors through treatment with the growth factors fibroblast growth factor 2 and epidermal growth factor. Glial progenitors can give rise to either astrocytes or oligodendrocytes. BDNF: Brain-derived neurotrophic factor; GDNF: Glial cell line-derived neurotrophic factor.
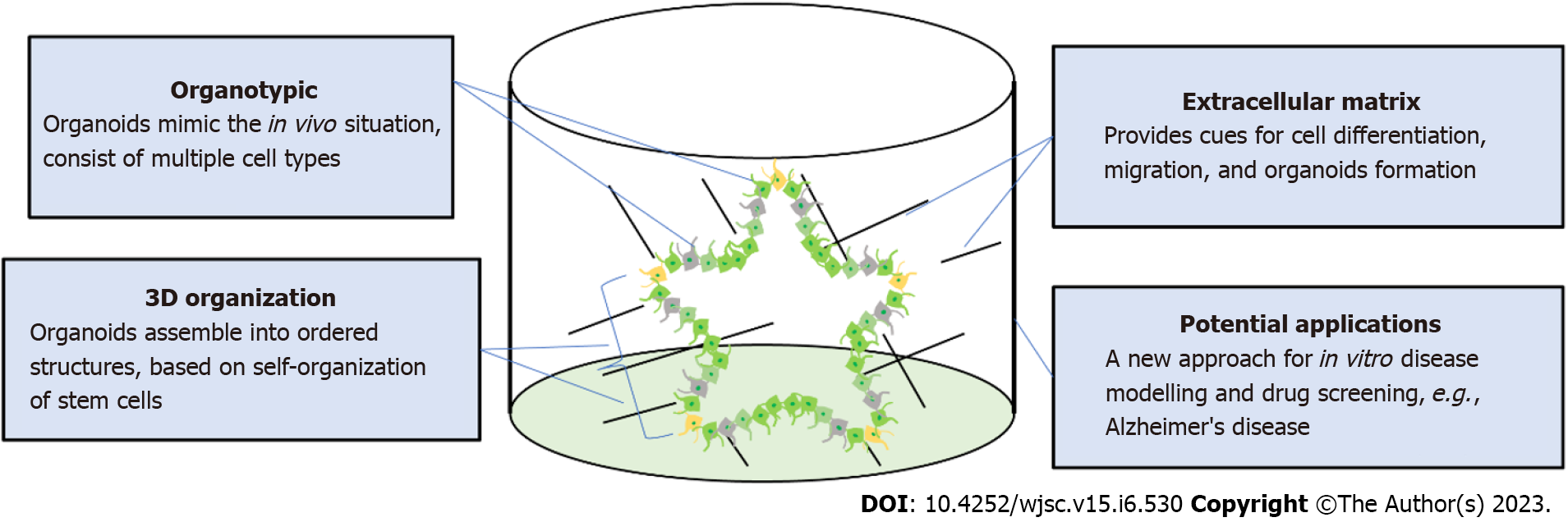
Figure 2 Self-organization of brain organoids.
Human brain organoids are generated based on the self-organizing properties of stem cells. Organoids usually contain multiple cell types including mature neurons and immature neural progenitors. The key to organoid regeneration is the extracellular matrix that is used to support stem cell growth and differentiation. Brain organoids have been widely utilized to model neurological pathology in disease such as Alzheimer’s disease and microcephaly.
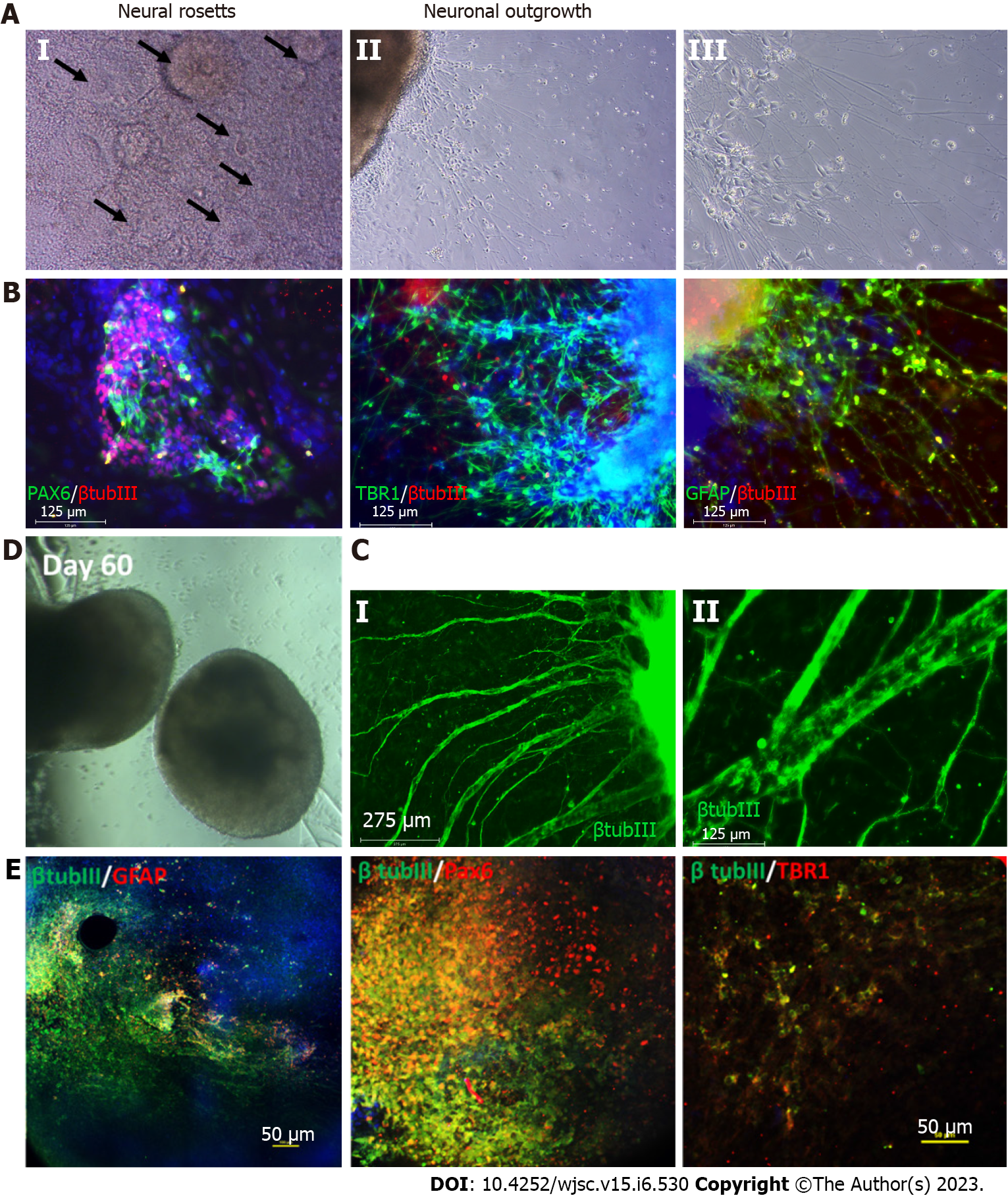
Figure 3 Characterization of cortical organoids for neural and astrocyte marker expression.
A: Brightfield images showing the neural rosettes and neuronal outgrowth from the organoids replated to an attachment plate at day 35 of differentiation; B: Resulting immunocytochemistry analysis of neural marker paired box 6 (PAX6), cortical deep layer VI marker T-box brain transcription factor 1 (TBR1), astrocyte marker glial fibrillary acidic protein (GFAP) co-stained with common neural marker β tubulin III, scale bar 125 µm; C: Immunostaining at later stage of the replating showing thick axon like extensions from the organoids, scale bar: 275 µm; D: Brightfield images of the day 60 cortical organoids; E: Confocal images of the day 60 organoids showing astrocyte marker GFAP, neural marker PAX6, cortical deep layer VI marker TBR1 co-stained with common neural marker β tubulin III, scale bar: 50 µm.
- Citation: Yan YW, Qian ES, Woodard LE, Bejoy J. Neural lineage differentiation of human pluripotent stem cells: Advances in disease modeling. World J Stem Cells 2023; 15(6): 530-547
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/530.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.530