Copyright
©The Author(s) 2023.
World J Stem Cells. Mar 26, 2023; 15(3): 52-70
Published online Mar 26, 2023. doi: 10.4252/wjsc.v15.i3.52
Published online Mar 26, 2023. doi: 10.4252/wjsc.v15.i3.52
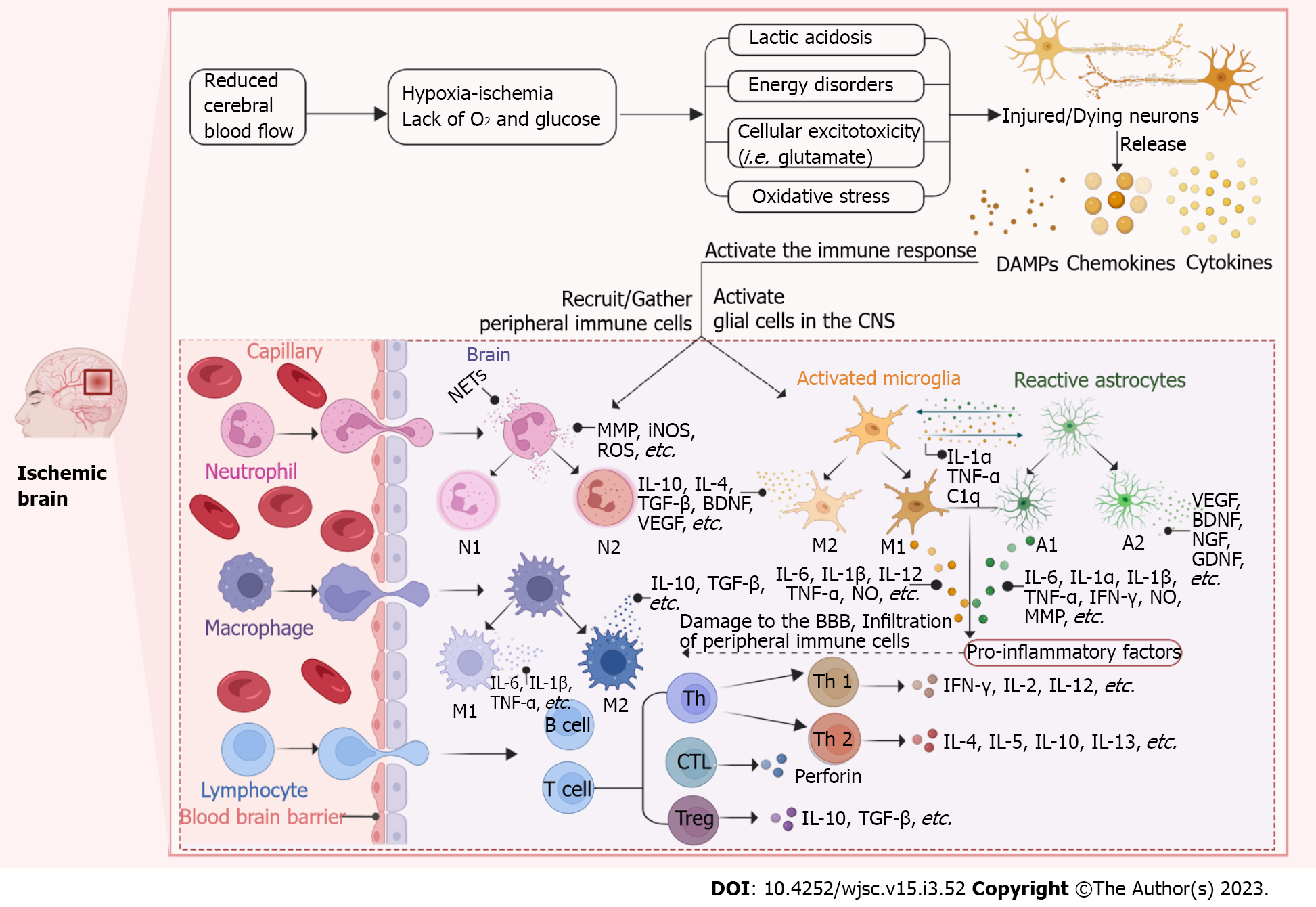
Figure 1 Schematic representation of the immune response after ischemic stroke.
After ischemic stroke (IS), the blood-brain barrier (BBB) is disrupted and the central nervous system (CNS) and the peripheral immune system are able to interact with each other. The cerebral blood flow is significantly reduced immediately after IS, which limits the availability of glucose and oxygen. The initial ischemic event leads to energy disturbances, acidosis, cellular excitotoxicity and oxidative stress ultimately resulting in neuronal damage or death and subsequent activation of the immune response. Dying/dead neurons release “danger signals” such as danger associated molecular patterns (DAMPs), cytokines and chemokines to recruit and activate peripheral immune cells (neutrophils, macrophages and lymphocytes) and activate glial cells (microglia and astrocytes) in the central CNS. Activated glial cells release a range of cytokines and chemokines that also recruit peripheral immune cells into the brain parenchyma and further destroy the BBB. Activated cells polarize into different cell phenotypes or subtypes to secrete pro-inflammatory or anti-inflammatory factors that act to damage or protect. (Figure created with BioRender.com). DAMP: Danger associated molecular patterns; CNS: Central nervous system; NET: Neutrophil extracellular trap; MMP: Matrix metalloproteinases; iNOS: Influx activates nitric oxide synthase; ROS: Reactive oxygen species; IL: Interleukin; TGF-β: Transforming growth factor-β; BDNF: Brain-derived neurotrophic factor; VEGF: Vascular endothelial growth factor; TNF-α: Tumor necrosis factor-α; NGF: Nerve growth factor; GDNF: Glial cell-derived neurotrophic factor; IFN-γ: Interferon-γ; BBB: Blood-brain barrier; CTL: Cytotoxic T lymphocyte; C1q: Component subunit 1q.
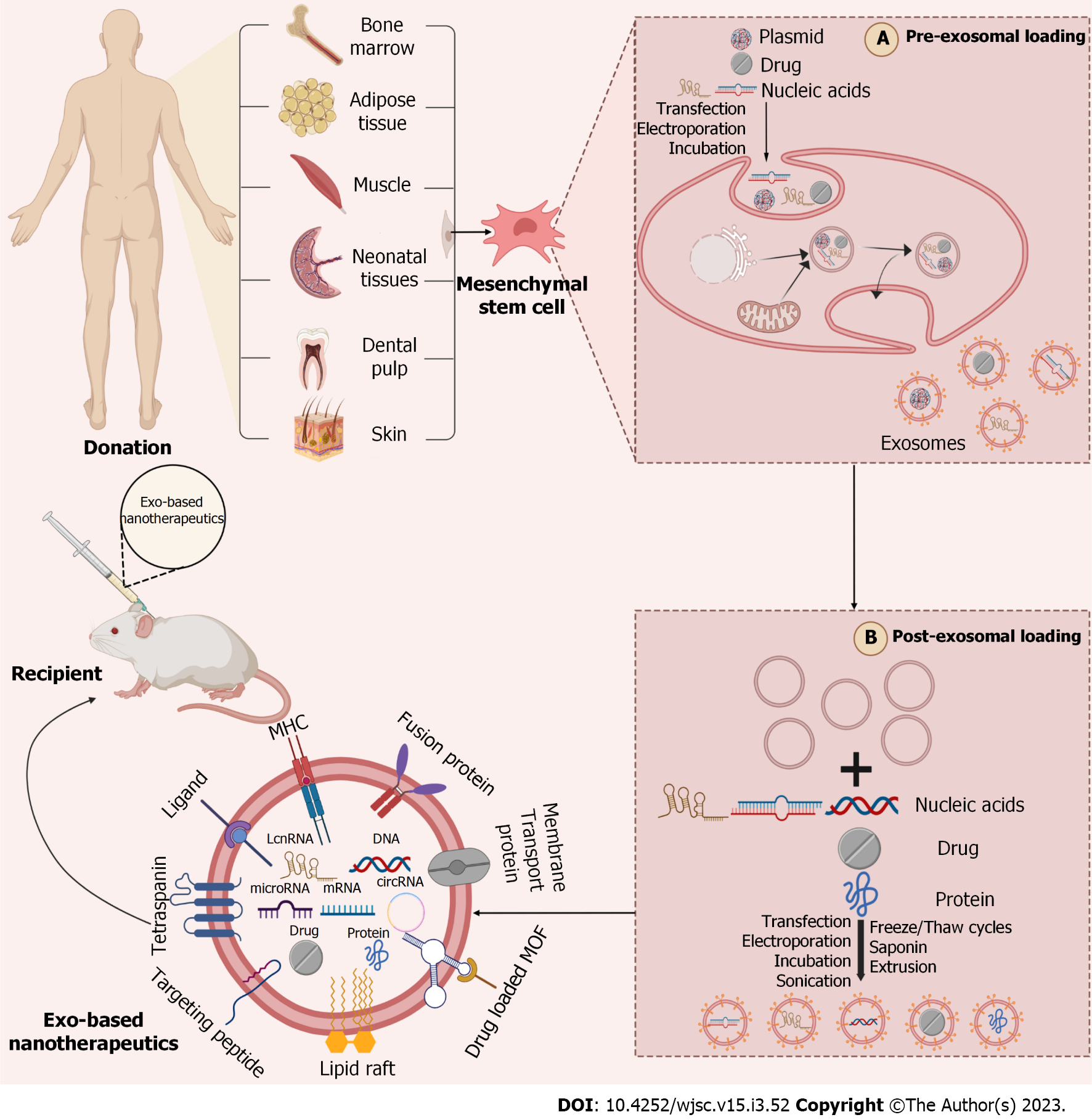
Figure 2 Strategies to enhance the targeting of mesenchymal stem cell-derived exosomes.
Multiple strategies to be done before exosomes can be successfully translated into new technologies to improve the targeting ability of donor cells and therapeutic efficacy of chemical and biomolecular drugs. Current preclinical studies are focused on parental cells' modification (pre-isolation) (A); manipulation of exosome (post-isolation) (B). (Figure created with BioRender.com).
- Citation: Shan XQ, Luo YY, Chang J, Song JJ, Hao N, Zhao L. Immunomodulation: The next target of mesenchymal stem cell-derived exosomes in the context of ischemic stroke. World J Stem Cells 2023; 15(3): 52-70
- URL: https://www.wjgnet.com/1948-0210/full/v15/i3/52.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i3.52