Copyright
©The Author(s) 2022.
World J Stem Cells. Feb 26, 2022; 14(2): 146-162
Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.146
Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.146
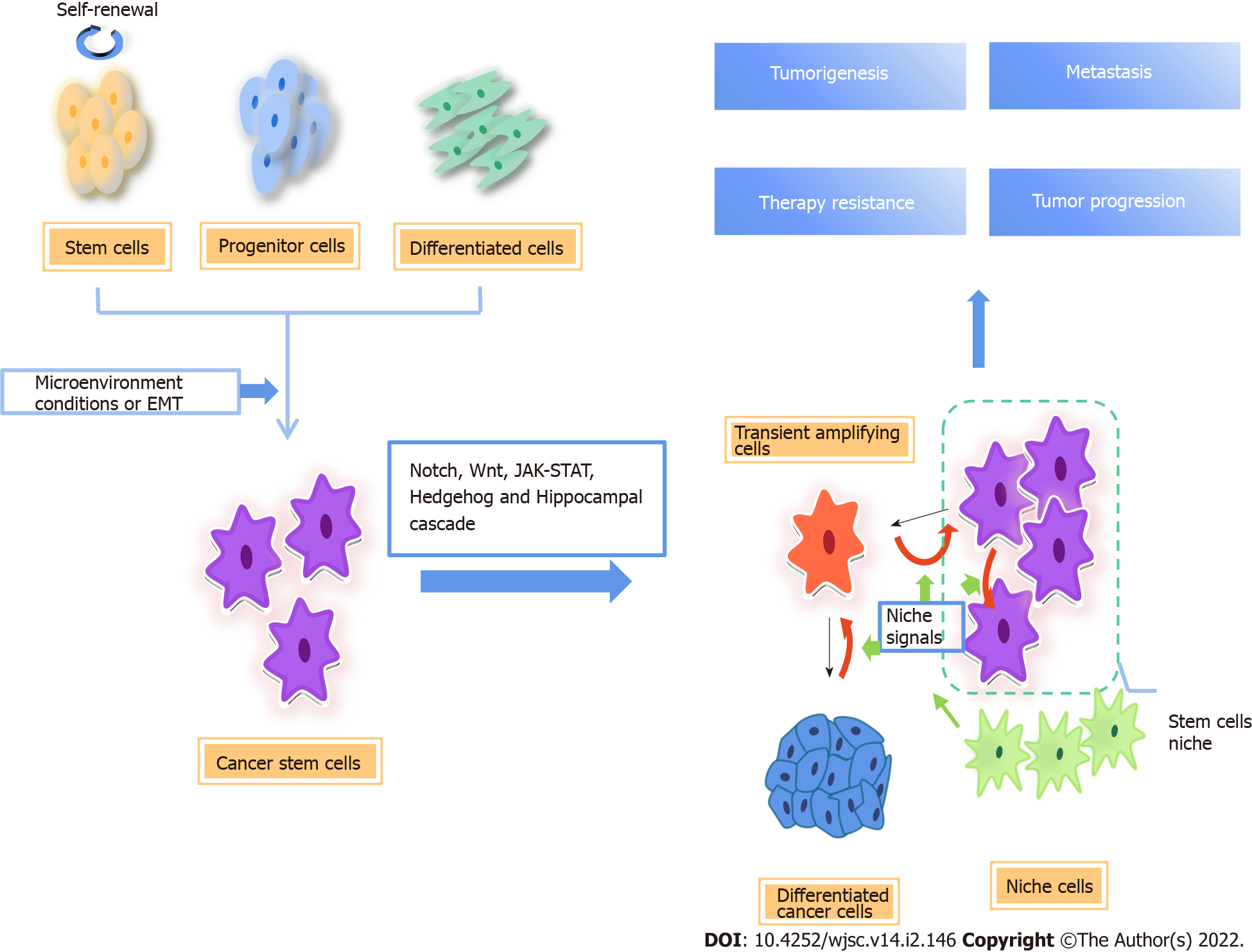
Figure 1 Origin of cancer stem cells and regulatory pathways involved.
There are two possible origins of cancer stem cells (CSCs), one is normal stem cells/progenitor cells, and the other is fully differentiated cells. CSCs are closely related to tumor microenvironmental factors. In the process of epithelial-mesenchymal transformation, cancer cells acquire stem cell-like characteristics. The differentiation direction of CSC progeny is determined by niche signal, and the available niche space determines the number of progeny stem cells. When there is no space available in the niche, the stem cells divide into transient amplifying (TA) cells, which divide and differentiate rapidly. At the same time, niche cells reprogram TA cells and differentiated cells into CSCs by niche signals[7]. CSCs are important subsets of tumor cells, which are regulated by a variety of signal pathways, including Notch, Wnt/β-catenin, Hippo, and Hedgehog signaling, which are the main causes of cancer initiation, progression, metastasis, therapy resistance, and relapse. EMT: Epithelial-to-mesenchymal transition.
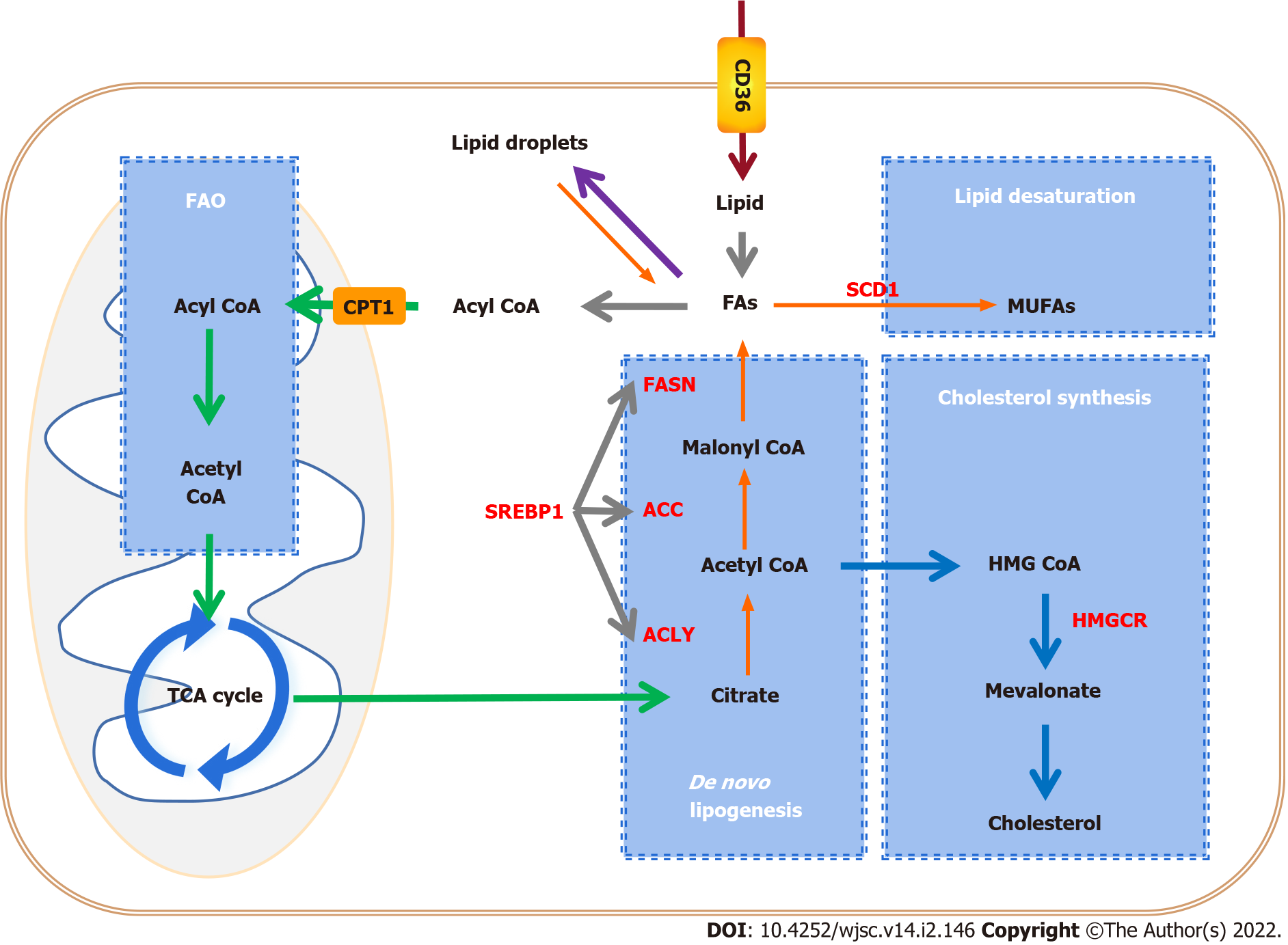
Figure 2 Alteration of lipid metabolic pathways in tumors and cancer stem cells.
Cancer stem cells (CSCs) enhance lipid metabolic activities, such as fatty acid synthesis, fatty acid oxidation, and lipid storage, to promote self-renewal and proliferation. Key enzymes that control lipid metabolism (red letters) are considered to be ideal therapeutic targets for CSCs. CPT1: Carnitine palmitoyl-transferase 1; FAO: Fatty acid oxidation; TCA cycle: Tricarboxylic acid cycle; CD36: Cluster of differentiation 36; FA: Fatty acid; FASN: Fatty acid synthase; ACC: Acetyl-CoA carboxylase; ACLY: ATP citrate lyase; SREBP1: Sterol-regulatory element binding protein 1; SCD1: Stearoyl-CoA desaturase 1; MUFA: Monounsaturated fatty acid; HMGCR: 3-hydroxy-3-methylglutaryl coenzyme A reductase.
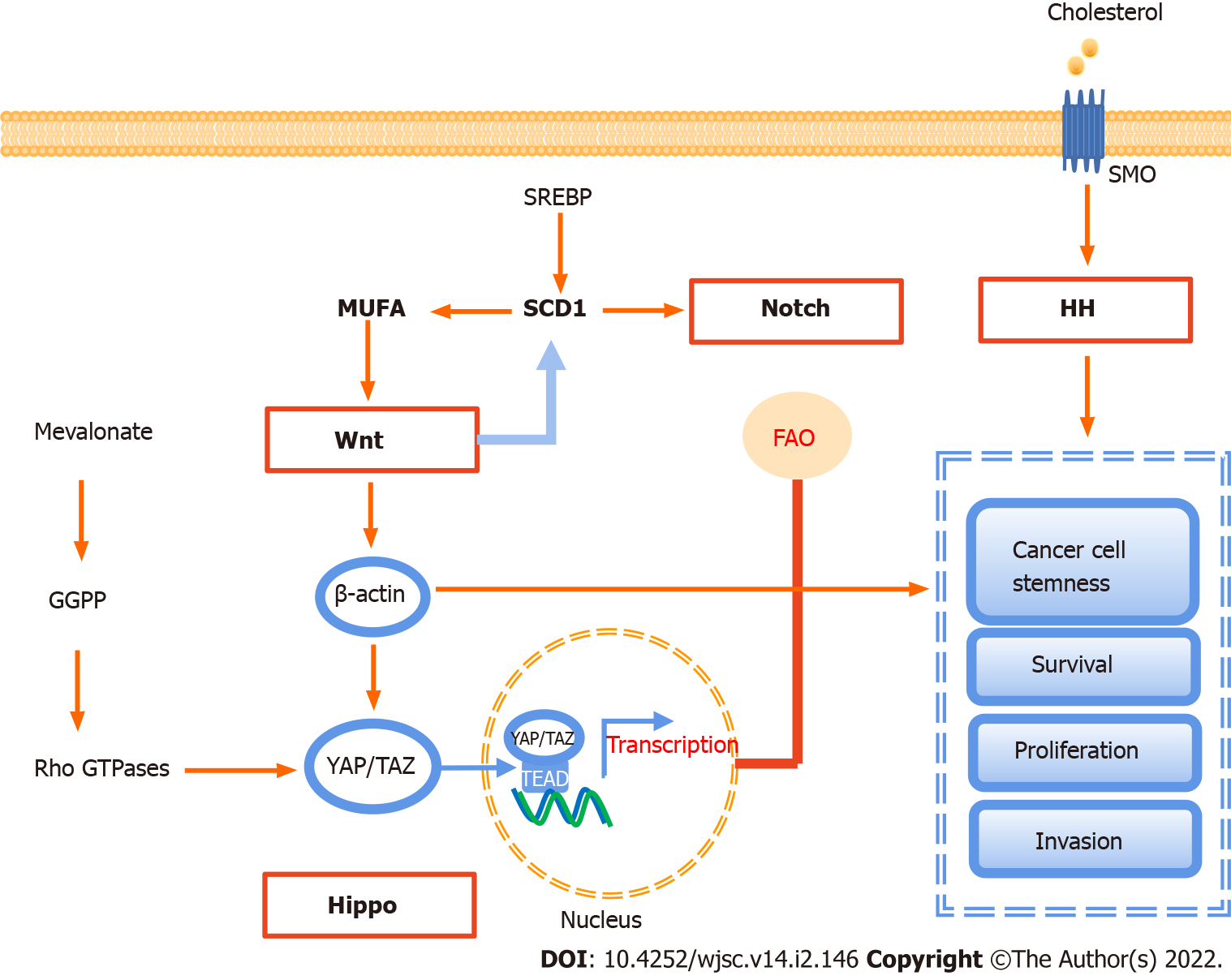
Figure 3 Signaling pathways involved in lipid metabolism in cancer stem cells.
There are four major signaling pathways, including Notch, Wnt, Hippo, and Hedgehog signaling, involved in lipid metabolism to maintain cell stemness, and sustain their survival, proliferation, and invasion. GGPP: Geranylgeranyl pyrophosphate; MUFA: Monounsaturated fatty acids; YAP: Yes-associated protein; TAZ: Transcriptional co-activator with PDZ-binding motif; SREBP: Sterol regulatory element-binding protein; SCD1: Stearyl coenzyme A desaturase 1; TEAD: Transcriptional enhanced associate domain; FAO: Fatty acid oxidation; SMO: Smoothened; HH: Hedgehog; SMP: Scalp micropigmentation.
- Citation: Wang SY, Hu QC, Wu T, Xia J, Tao XA, Cheng B. Abnormal lipid synthesis as a therapeutic target for cancer stem cells. World J Stem Cells 2022; 14(2): 146-162
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/146.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.146