Copyright
©©The Author(s) 2022.
World J Stem Cells. Jan 26, 2022; 14(1): 54-75
Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.54
Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.54
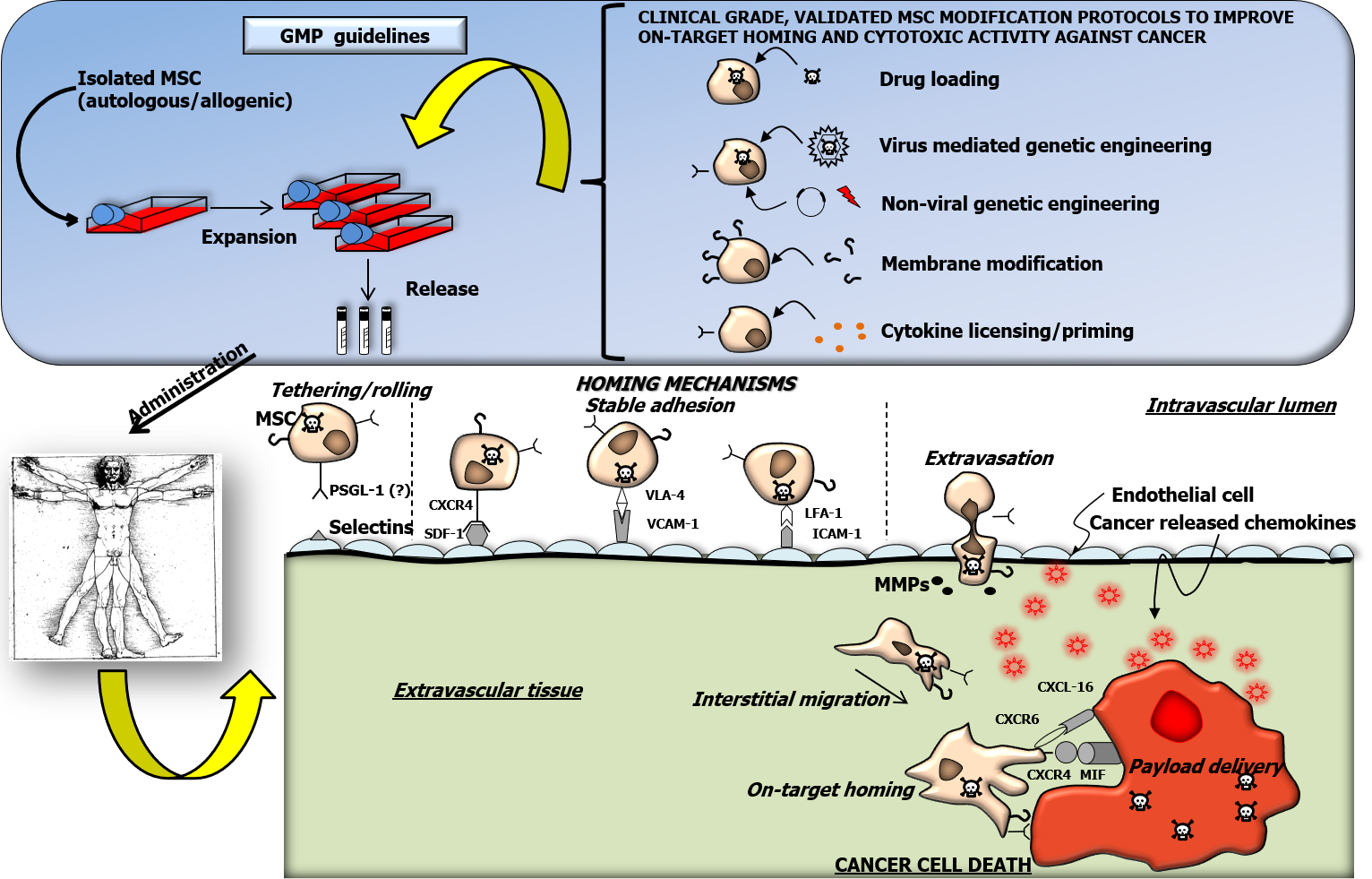
Figure 1 Graphic summary.
A graphic simplified summary of mesenchymal stem stromal cells (MSC) ex vivo handling and of possible cell modification strategies under good manufacturing practice regulations is reported in Figure 1. In particular, the possibility of improving MSC homing capacity through viral/non-viral genetic engineering, membrane modification and cytokine licensing/priming is reported. In parallel, genetic engineering and direct drug loading are illustrated as a mean of inducing a cytotoxic phenotype in MSC. In the lower section of the figure, relevant molecular mechanisms controlling distinct phases (tethering/rolling, firm adhesion, extravasation, interstitial migration) of the homing process to the cancer mass, potentially occurring after systemic administration of modified MSC to human patients are illustrated. PSGL-1: P-selectin glycoprotein ligand-1; CXCR4: C-X-C chemokine receptor type 4; SDF-1: Stromal derived factor-1; VLA-4: Very late antigen-4; VCAM-1: Vascular cell adhesion molecule 1; LFA-1: Lymphocyte function-associated antigen 1; ICAM-1: Intercellular adhesion molecule 1; MMPs: Metalloproteases; CXCR6: C-X-C chemokine receptor type 6; CXCL16: C-X-C motif ligand 16; MIF: Macrophage migration inhibitory factor; MSC: Mesenchymal stem stromal cells; GMP: Good manufacturing practice.
- Citation: Vicinanza C, Lombardi E, Da Ros F, Marangon M, Durante C, Mazzucato M, Agostini F. Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications. World J Stem Cells 2022; 14(1): 54-75
- URL: https://www.wjgnet.com/1948-0210/full/v14/i1/54.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i1.54