Copyright
©The Author(s) 2022.
World J Stem Cells. Jan 26, 2022; 14(1): 1-40
Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.1
Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.1
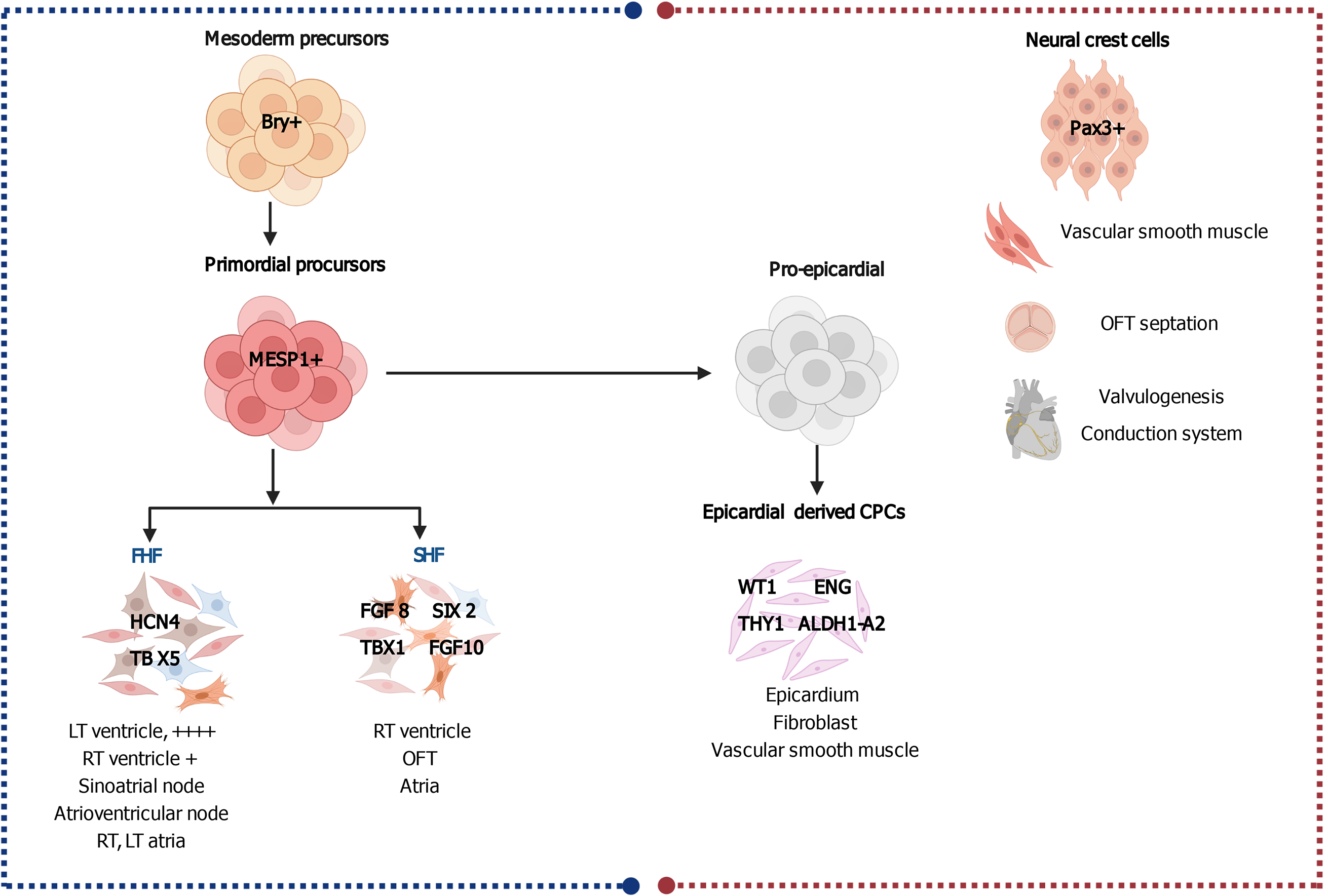
Figure 1 Embryonic cardiac progenitors, Brachyury-positive mesoderm precursors and Pax3+ neural crest cells.
Brachyury (Bry+) mesoderm precursors give rise to the mesoderm posterior 1+ primordial precursors, which are the origin of the first heart field, second heart field, and proepicardial progenitors, each population of which is responsible for the development of different parts in the heart. Pax3+ neural crest cells are responsible for the development of vascular smooth muscle, outflow tract, valves and the conductive system. Progenitors are tagged with their specific markers. Created with BioRender.com. CPC: Cardiac progenitor cell; LT: Left; RT: Right; FHF: First heart field; SHF: Second heart field; OFT: Outflow tract.
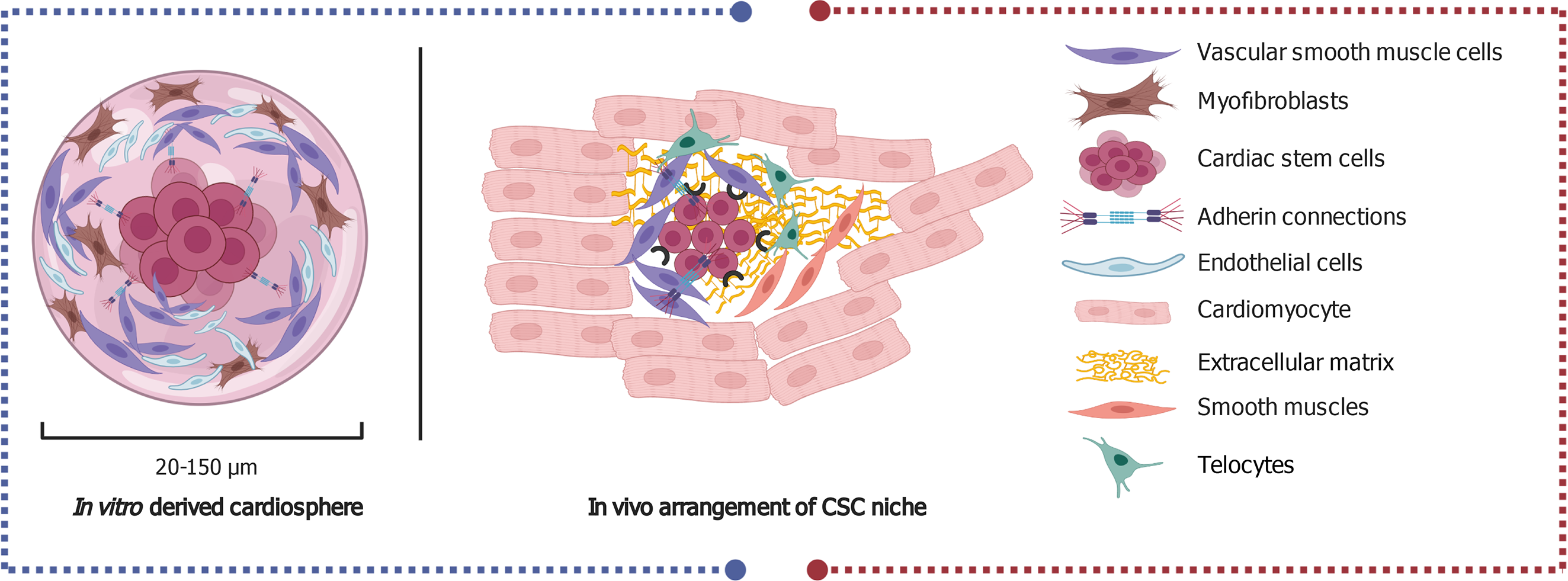
Figure 2 Invivo arrangement of the central cardiac stem cells and the surrounding cells that comprise the niche (right side) and the in vitro derived cardio spheres (left side).
The key delineates the types of cells identified in the niche and cardio spheres. Created with BioRender. CSC: Cardiac stem cell.
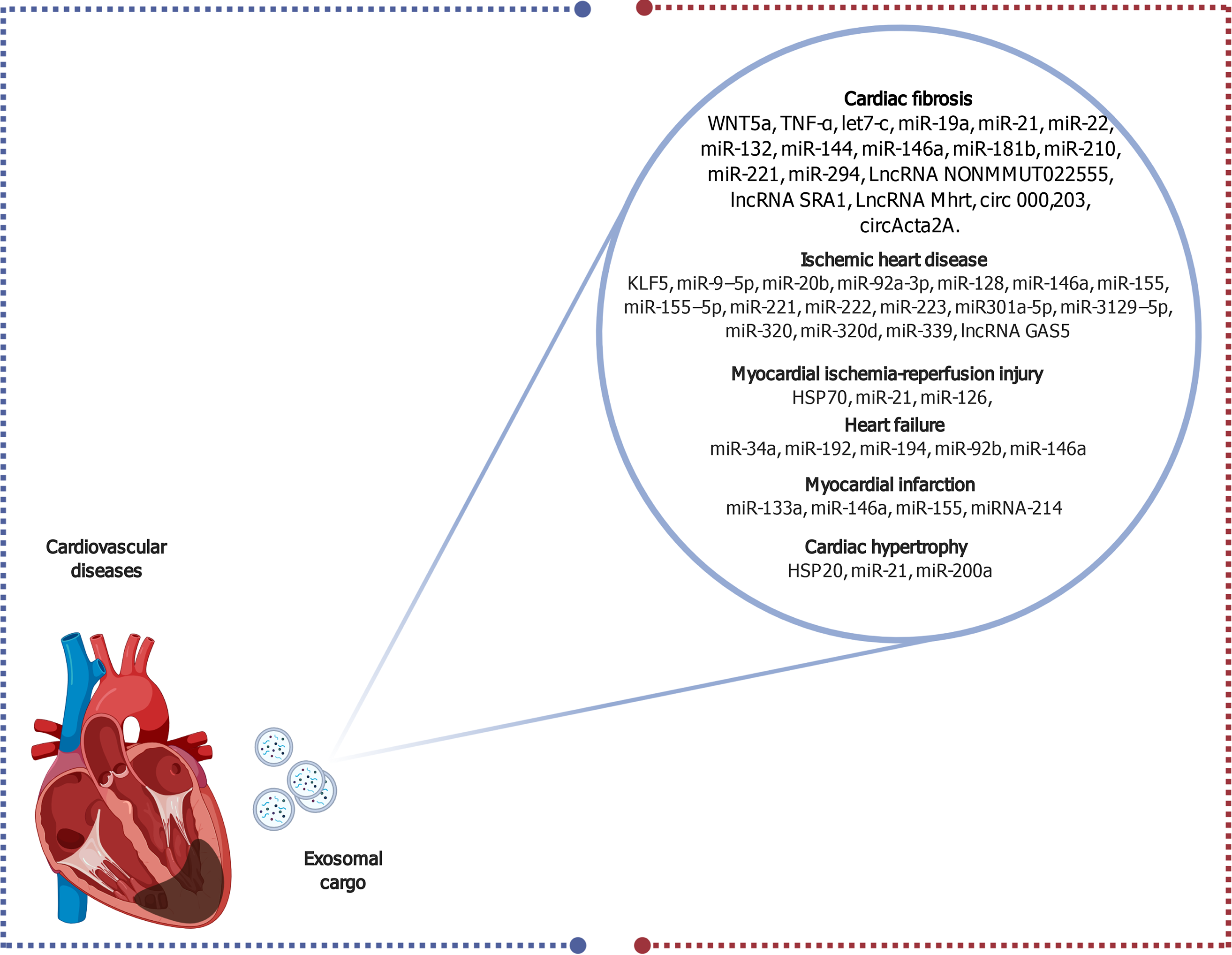
Figure 3 Schematic diagram elucidating the diverse exosomal contents that serve as biomarkers for several cardiovascular diseases.
Created with BioRender.com. HSP: Heat shock protein; lncRNA: Long non-coding RNA; miR: MicroRNA.
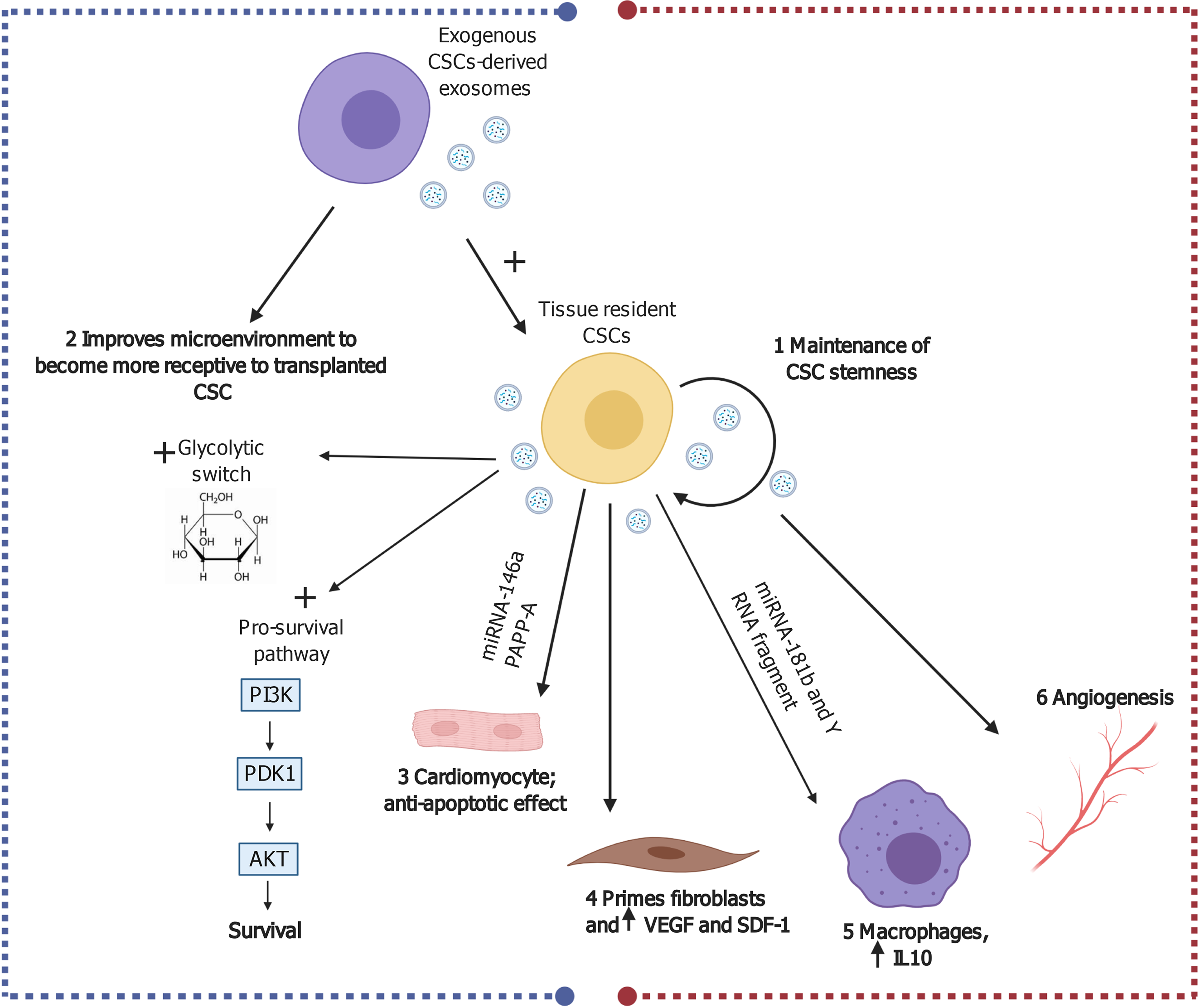
Figure 4 Possible cardiac reparative effects of cardiac stem cell/cardiosphere-derived cell-derived exosomes in myocardial ischemia and ischemia/reperfusion injury.
Created with BioRender.com. CSC: Cardiac stem cell; IL: Interleukin; IR: Ischemia/reperfusion; miRNA: MicroRNA; PI3K: Phosphoinositide 3-kinase; SDF-1: Stromal cell-derived factor 1; VEGF: Vascular endothelial growth factor.
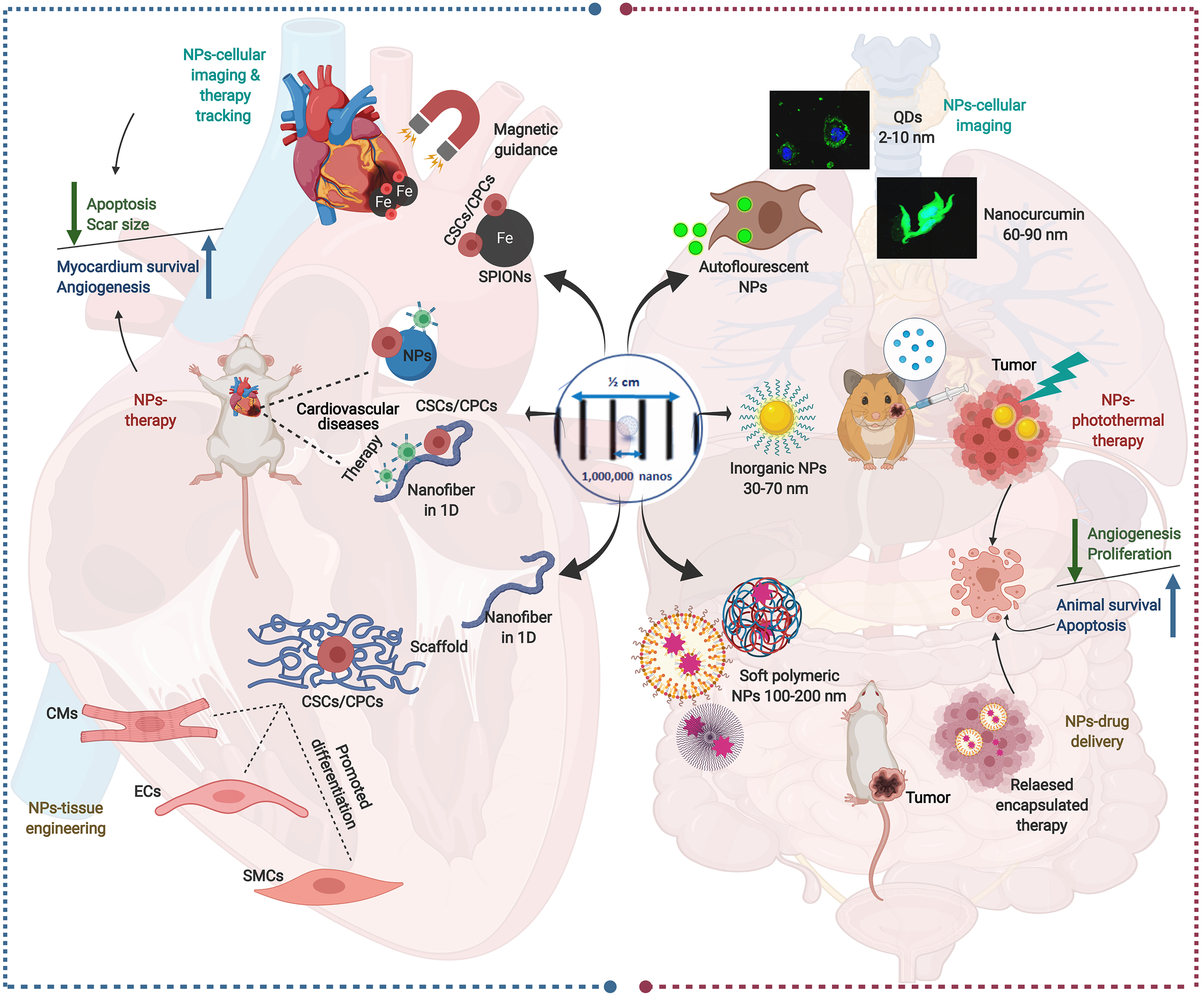
Figure 5 Schematic presentation of multiple nanoparticles-based paradigms in nanomedicine (right side) and their mirror images in nanoparticle-assisted cardiac stem cell interventions (left side).
In the right side, the auto-luminescence of quantum dots and some polymeric natural nanoparticles (NPs) as nanocurcumin qualifies them to be used primarily in tracking and diagnostic imaging. Meanwhile, inorganic NPs have been extensively investigated in cancer treatment as photothermal therapy due to the plasmonic resonance of their outer electron. For drug delivery purposes, soft NPs, such as liposomes, polymicelles, and dendrimers, are used due to their flexibility and biophysical interaction with components of the cell membrane, which enable them to penetrate biological membranes. In the left side, the impact of different NPs on cardiac stem cell (CSC)-based therapy are shown. The superparamagnetic iron oxide NPs are used for tracking and imaging of the CSCs-therapy, while nanofibers are used either as a dual delivery system for CSCs and therapy. Moreover, nanofiber scaffold holding the CSCs is used in tissue engineering to assist CSCs’ differentiation. The confocal images of NPs-cellular imaging are reproduced with permission[196]. Created with BioRender.com. NPs: Nanoparticles; SMCs: Smooth muscle cells; CMs: Cardiomyocytes; ECs: Endocardial cells; CSCs: Cardiac stem cells; CPCs: Cardiac progenitor cells; SPION: Superparamagnetic iron oxide NP.
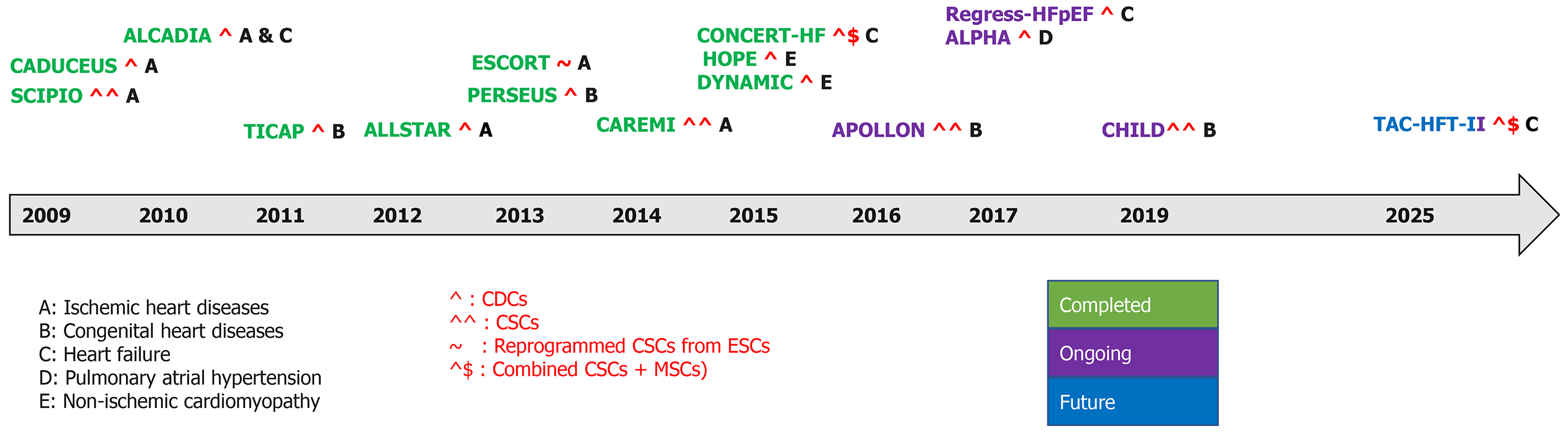
Figure 6 Timeline of completed, ongoing, and future clinical trials in cardiovascular diseases.
CDCs: Cardiosphere-derived cells; CSCs: Cardiac stem cells; ESCs: Embryonic stem cells; MSCs: Mesenchymal stem cells.
- Citation: Mehanna RA, Essawy MM, Barkat MA, Awaad AK, Thabet EH, Hamed HA, Elkafrawy H, Khalil NA, Sallam A, Kholief MA, Ibrahim SS, Mourad GM. Cardiac stem cells: Current knowledge and future prospects. World J Stem Cells 2022; 14(1): 1-40
- URL: https://www.wjgnet.com/1948-0210/full/v14/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i1.1