Copyright
©The Author(s) 2021.
World J Stem Cells. Jul 26, 2021; 13(7): 877-893
Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.877
Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.877
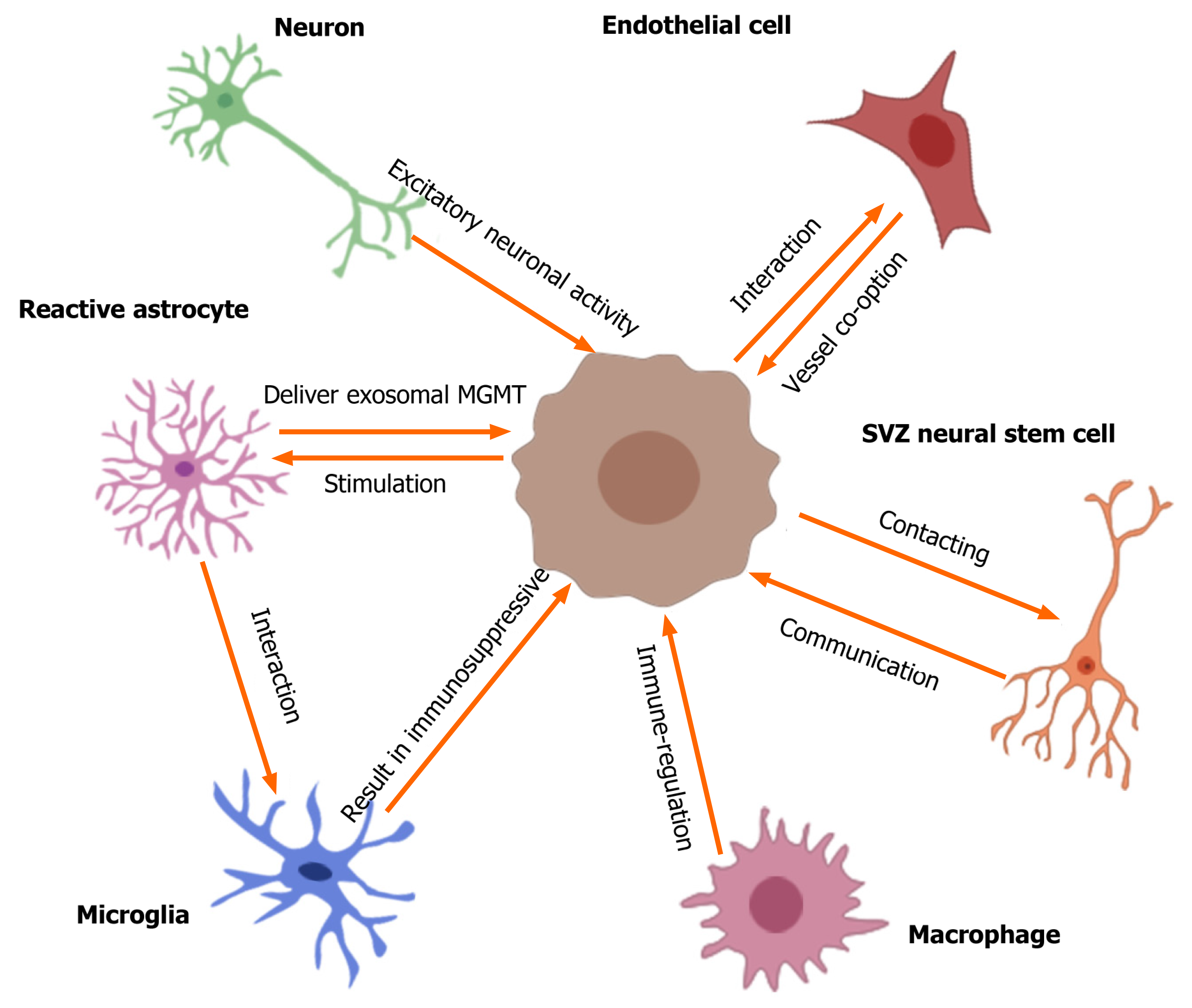
Figure 1 Crosstalk between gliomas and non-glioma cells in tumor microenvironment.
The tumor microenvironment of glioma contains many non-glioma cells around the tumor, such as neurons, astrocytes, oligodendrocytes, immune cells (microglia and macrophages), endothelial cells, stem cells, etc. Active neurons promote the growth of glioblastomas through secreted cytokine NLGN3. Excitatory neuronal activity mediates the neuron-glioma interactions mainly via the bona fide AMPA receptor-dependent neuron–glioma synapse. Glioma cells stimulate normal astrocytes into reactive astrocytes and then reactive astrocytes induce temozolomide-resistance of glioma cells via delivering exosomal MGMT. The interactions of astrocyte-microglia can regulate transcriptional re-programming of microglia, and microglia contribute to sustaining the immunosuppressive microenvironment in glioblastoma multiforme (GBM) for promoting tumor progression. Glioblastomas increase macrophage infiltration, and then infiltrated macrophages promote GBM survival and tumor angiogenesis via secreted cytokines. Olig2-positive glioma cells can be invaded by vessel co-option; the glioma-EC interactions restrict the anti-angiogenic therapy of gliomas. In addition, GBM contacting neural stem cells in the subventricular zone of the lateral ventricles result in a shorter overall survival of patients and increase early recurrence of gliomas. SVZ: Subventricular zone.
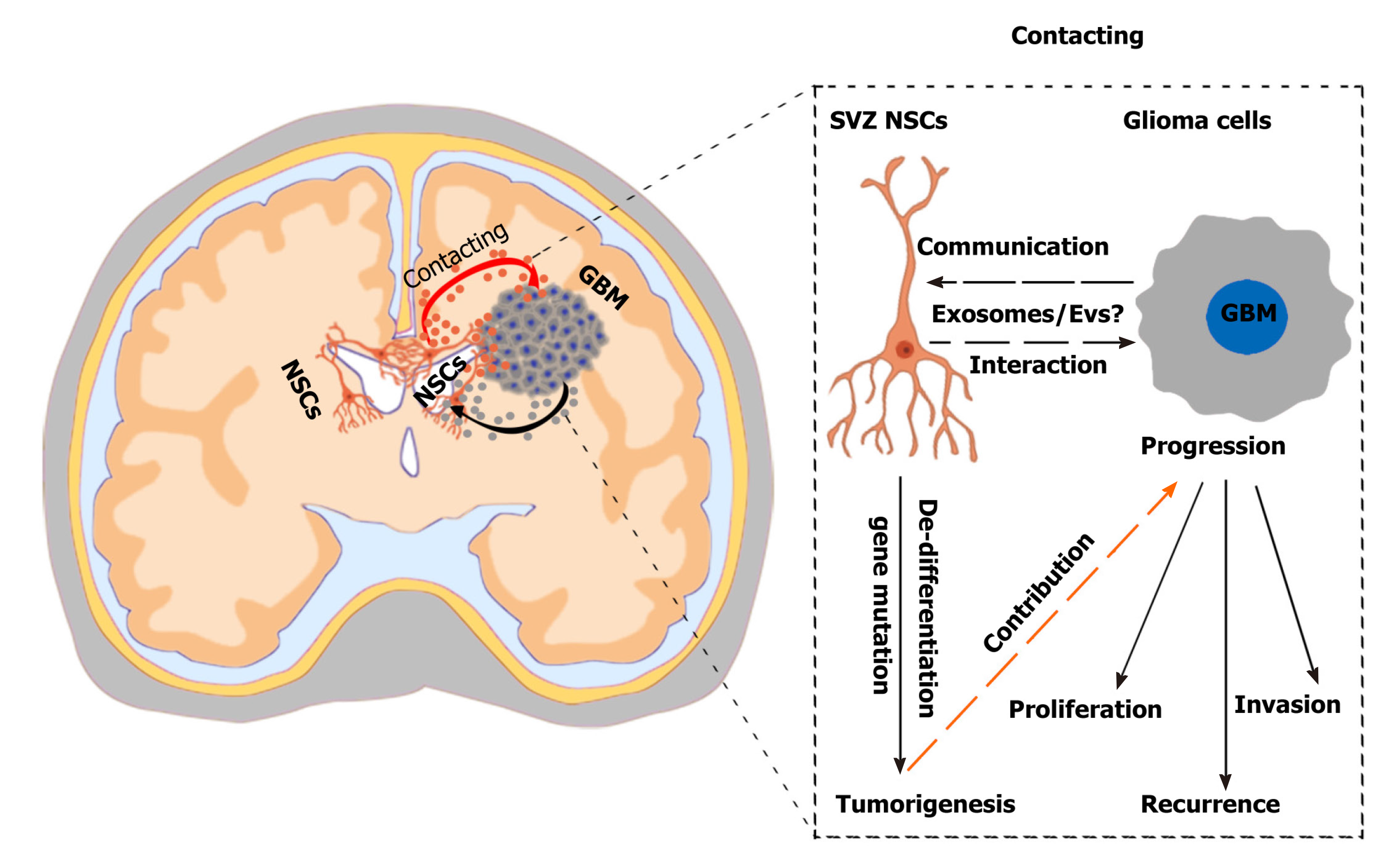
Figure 2 Challenges and effects of neural stem cells in the subventricular zone in glioma progression.
A hypothetical scenario shows the crosstalk between neural stem cells (NSCs) in the subventricular zone (SVZ) and glioblastoma multiformes and the effects of SVZ NSCs in glioma progression. SVZ NSCs can exert chemoattractant effects on glioma cells through secretion of chemoattractant complex factors [such as pleiotrophin, HSP90B, and secreted protein acidic and rich in cysteine (SPARC)/SPARC-like protein 1], and glioblastomas can induce SVZ NSCs to de-differentiate into tumor-promoting cells via glioblastoma-derived extracellular vesicles (EVs). The SVZ NSC-glioma interactions are mainly mediated by the secreted factors, especially EVs/exosomes. Understanding the biological mechanisms mediated by cytokines and EVs/exosomes will help to discover new therapeutic strategy. NSCs: Neural stem cells; SVZ: Subventricular zone; GBM: Glioblastoma multiforme.
- Citation: Zhang GL, Wang CF, Qian C, Ji YX, Wang YZ. Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J Stem Cells 2021; 13(7): 877-893
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/877.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.877