Copyright
©The Author(s) 2021.
World J Stem Cells. Nov 26, 2021; 13(11): 1714-1732
Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1714
Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1714
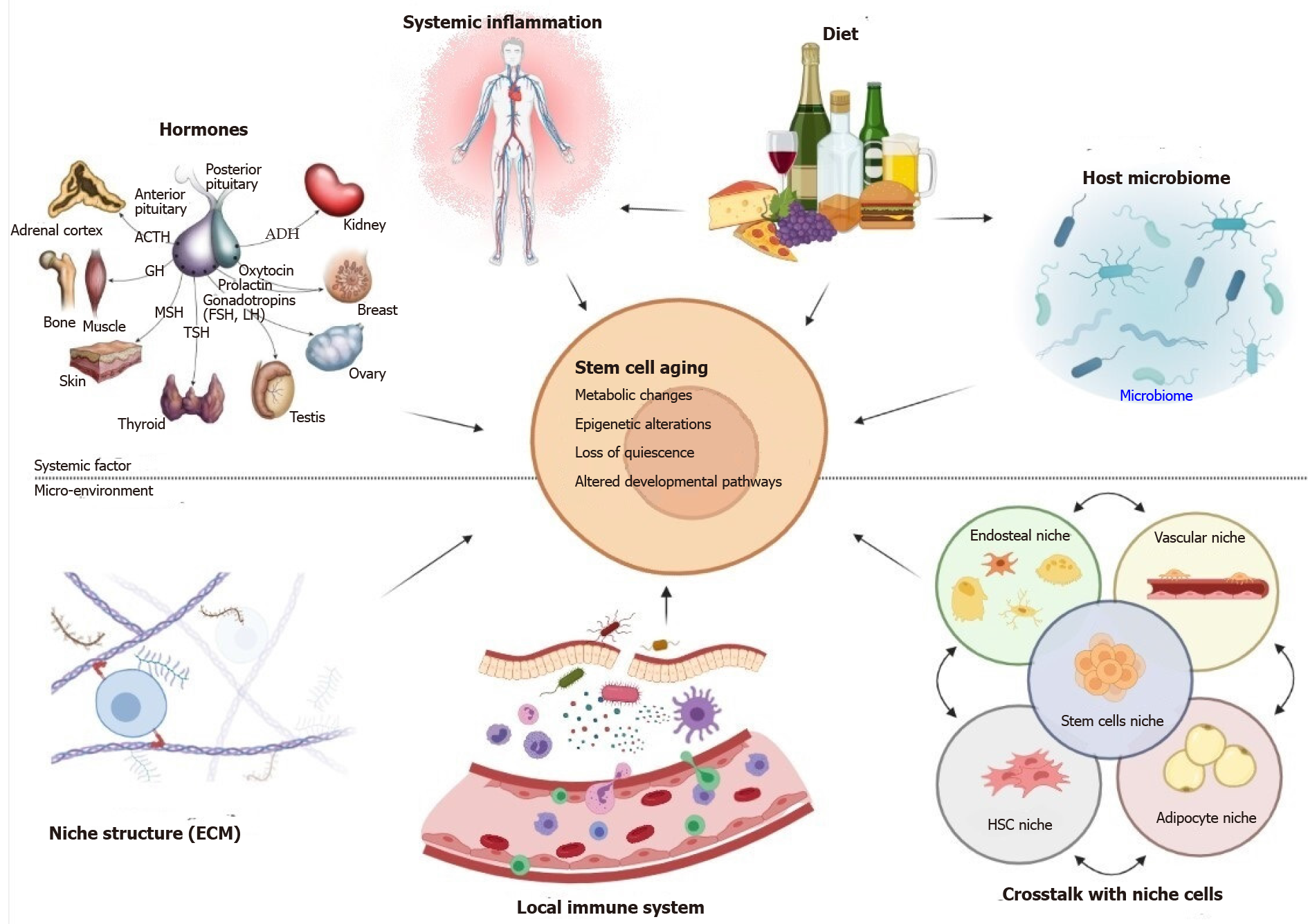
Figure 1 During the aging process, systemic influences and changes in the local microenvironment have an effect on stem cell activity.
Changes in systemic signals that control stem cell activity in multiple compartments are caused by a combination of physiological changes in hormone levels, increased inflammation, and the interplay between diet and the host microbiome. Locally, changes in the cellular and molecular composition of the stem cell niche caused by aging cause stem cells to be stimulated abnormally, influencing their quiescence, metabolism, and differentiation ability. Extrinsic signals often cause epigenetic modifications in stem cells, which may result in the aberrant reactivation of developmental pathways. ACTH: Adrenocorticotropin hormone; ADH: Antidiuretic hormone; GH: Growth hormone; MSH: Melanocyte-stimulating hormone; TSH: Thyroid-stimulating hormone; FSH: Follicular-stimulating hormone; LH: Luteinizing hormone; ECM: Extracellular matrix; HSC: Hematopoietic stem cell.
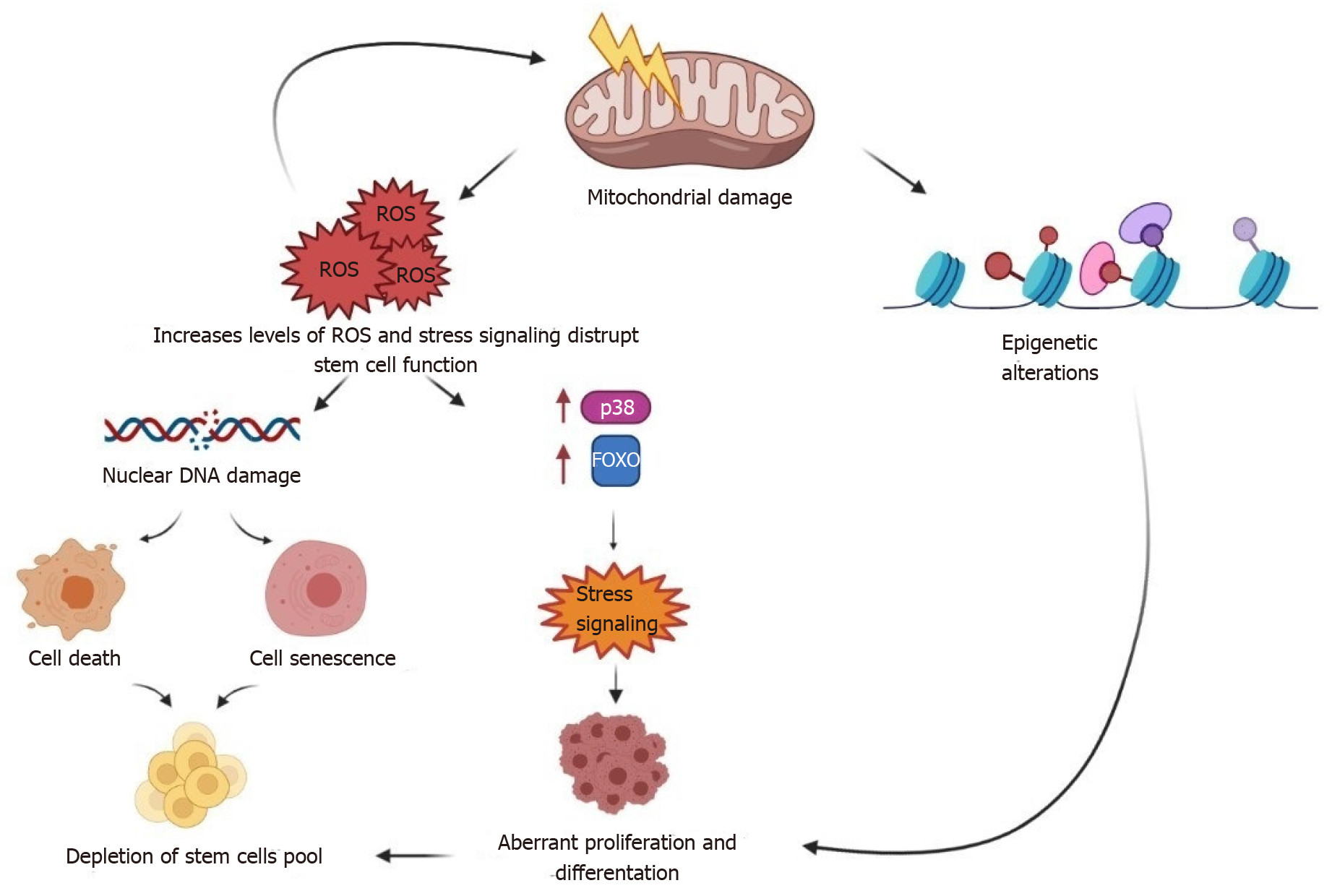
Figure 2 Molecular damage in stem cells as they age.
Cell-intrinsic changes in stem cells as they age are intricately linked. In aging cells, mechanisms that would normally ensure the clearance of damaged proteins (autophagy and proteasome-mediated degradation) work badly and cause the accumulation of toxicity and toxic protein aggregates. Excessive reactive oxygen species (ROS) are released by damaged mitochondria, causing further mitochondrial harm (including mitochondrial DNA damage). ROS accumulation causes nuclear DNA damage in aged stem cells, which is aggravated by DNA replication errors and defective DNA repair, leading to cell senescence and apoptosis. Although moderate production of ROS and other stresses are necessary for the regulation of stem cell proliferation and differentiation in normal physiology (at a young age), high levels of ROS trigger stress mediators (p38 and forkhead box protein, resulting in abnormal stem cell function. As a result, stem cell reserves are depleted, and self-renewal is impaired. ROS: Reactive oxygen species.
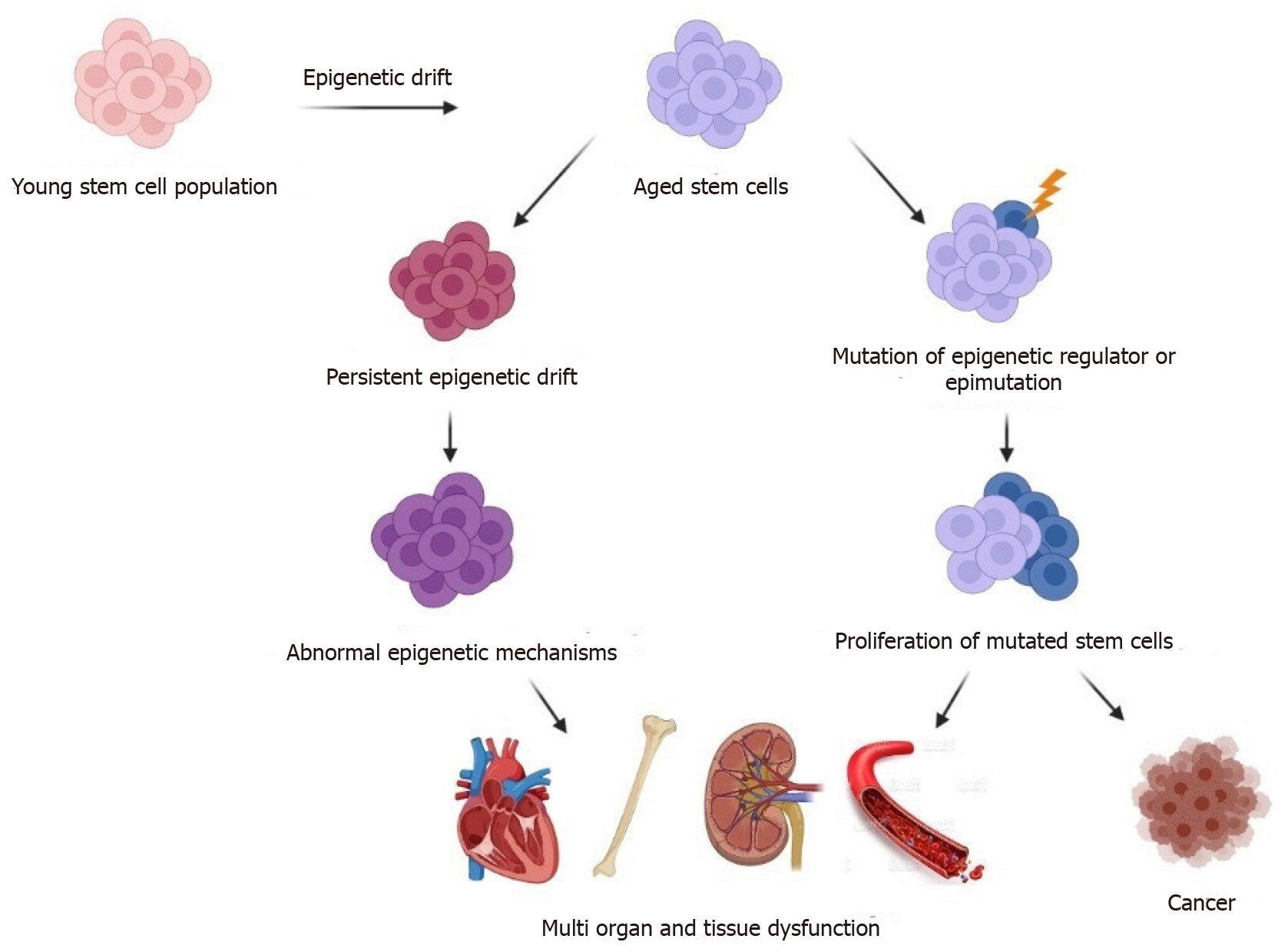
Figure 3 Adult stem cells are altered by epigenetic drift and clonal expansion as they age.
The epigenome of adult stem cells undergoes epigenetic drift after reproductive age, which may be caused by molecular disruption, changes in the stem cell niche, or abnormal activation of developmental programs. Depending on the stem cell compartment, DNA methylation and histone modification alterations that accumulate with age can be different and have different effects. Epigenetic drift enhances self-renewal and impairs differentiation of adult stem cells, according to new research. Epigenetic drift causes deregulation of epigenome-sensible gene pathways in certain stem cell compartments, which promotes stem cell dysfunction. Mutations in epigenetic modifiers (for example, DNMT3A), Tet methylcytosine dioxygenase 2, and putative Polycomb group protein ASXL1) have been found frequently in aged haematopoietic stem cells and can lead to the selection of mutant stem cells (blue) that gain clonal dominance, resulting in impairments in adult stem cell differentiation.
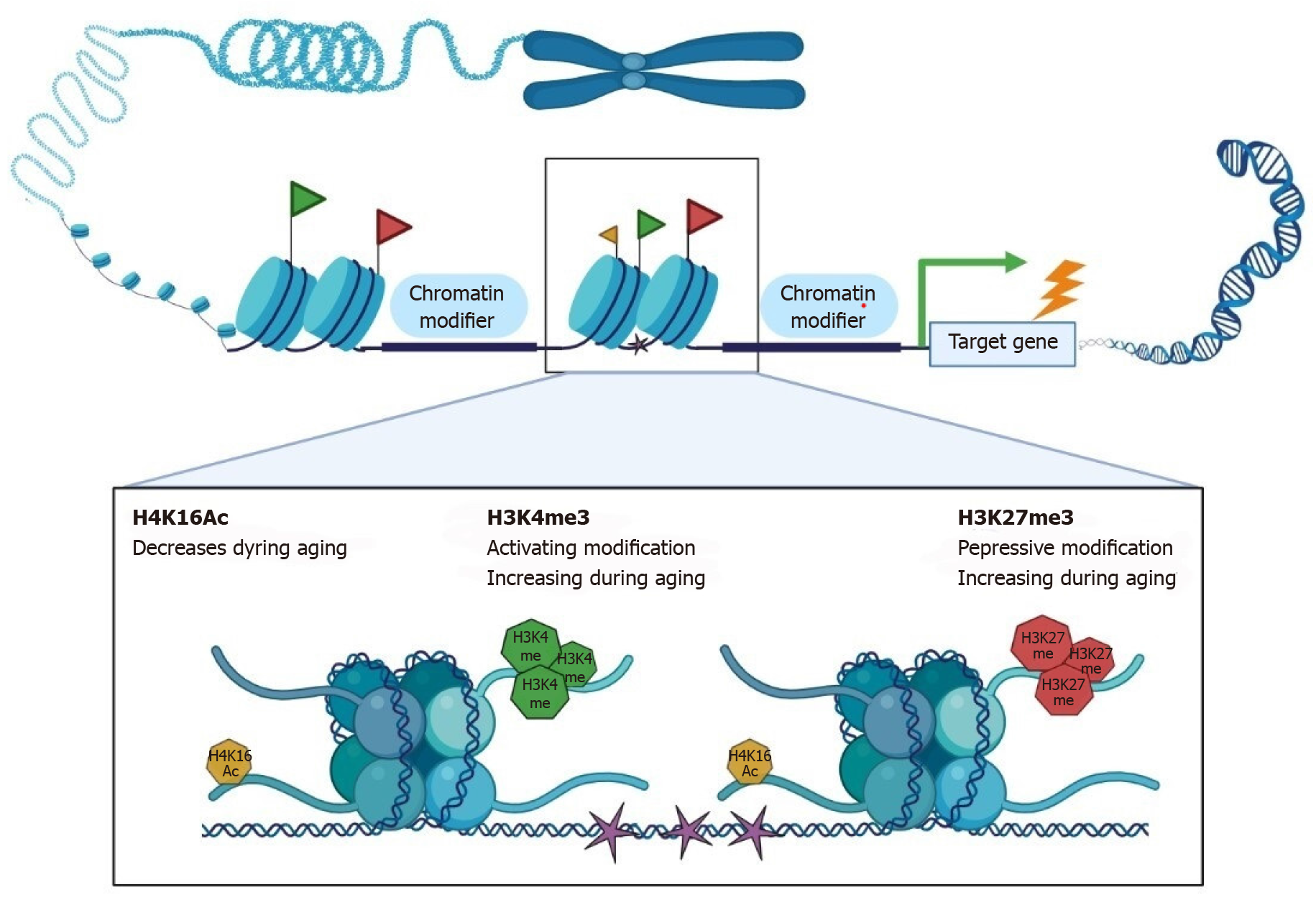
Figure 4 Changes in chromatin related to stem cell aging.
In hematopoietic stem cells, satellite cells, and other types of stem cells, changes in the structure and makeup of chromatin during aging have been studied. Differences in chromatin-modifying enzyme levels and distribution (blue oval shapes), histone modifications (green flag, activating; red flag, repressive), and DNA methylation patterns (pink star) are among these shifts. Regional loss of transcriptional silencing altered cell fate decisions, reduced activity, and cellular senescence are all consequences of these changes.
- Citation: Picerno A, Stasi A, Franzin R, Curci C, di Bari I, Gesualdo L, Sallustio F. Why stem/progenitor cells lose their regenerative potential. World J Stem Cells 2021; 13(11): 1714-1732
- URL: https://www.wjgnet.com/1948-0210/full/v13/i11/1714.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i11.1714