Copyright
©The Author(s) 2020.
World J Stem Cells. May 26, 2020; 12(5): 368-380
Published online May 26, 2020. doi: 10.4252/wjsc.v12.i5.368
Published online May 26, 2020. doi: 10.4252/wjsc.v12.i5.368
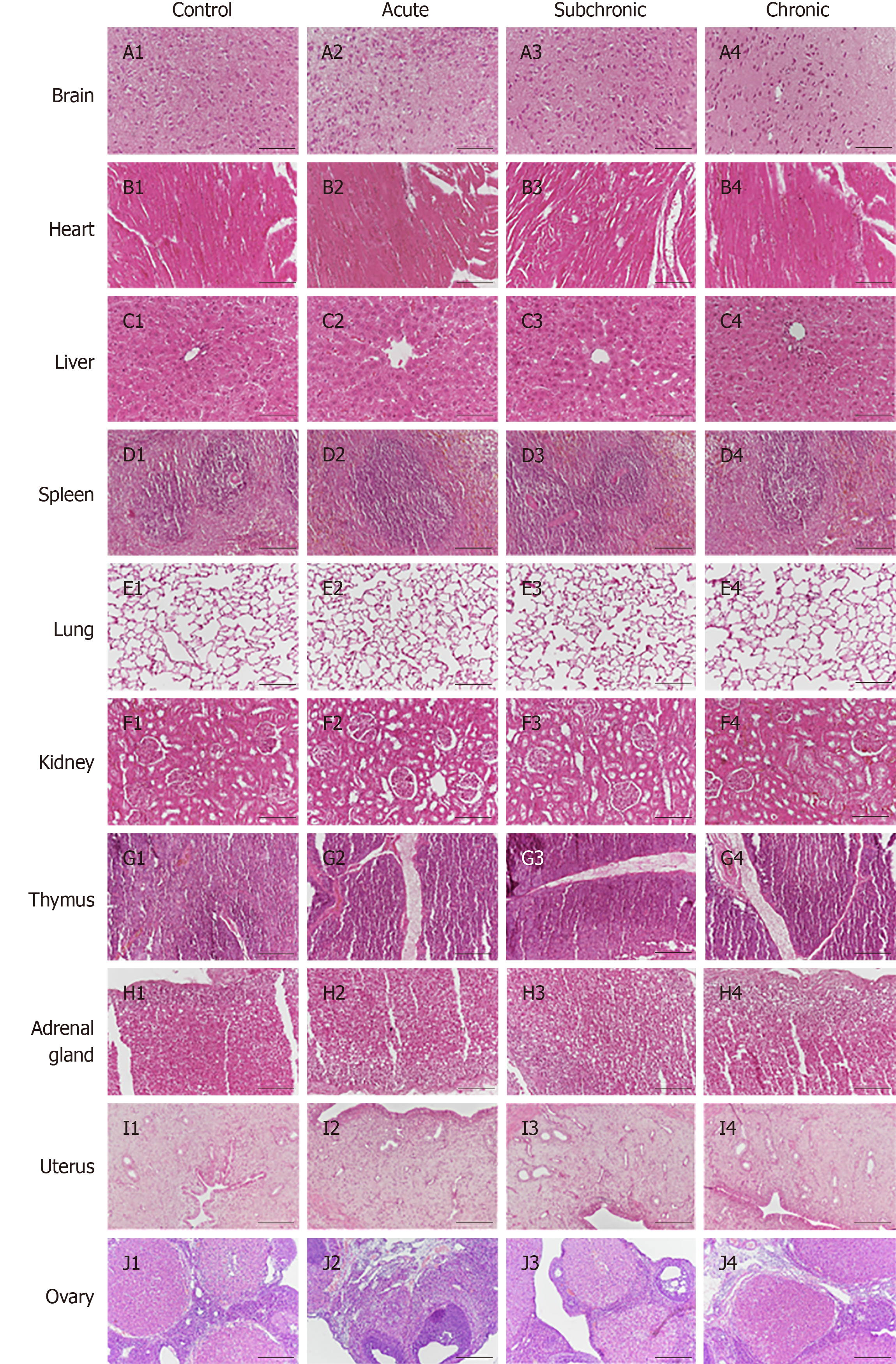
Figure 1 Histopathological analysis of intrauterine adhesion rats after menstrual-derived stromal stem cell treatment.
Representative H&E staining of various organs (brain, heart, liver, spleen, lungs, kidneys, thymus, adrenal glands, uterus, and ovaries). No structural changes or injuries were detected in theses organs. Scale bar = 100 μm.
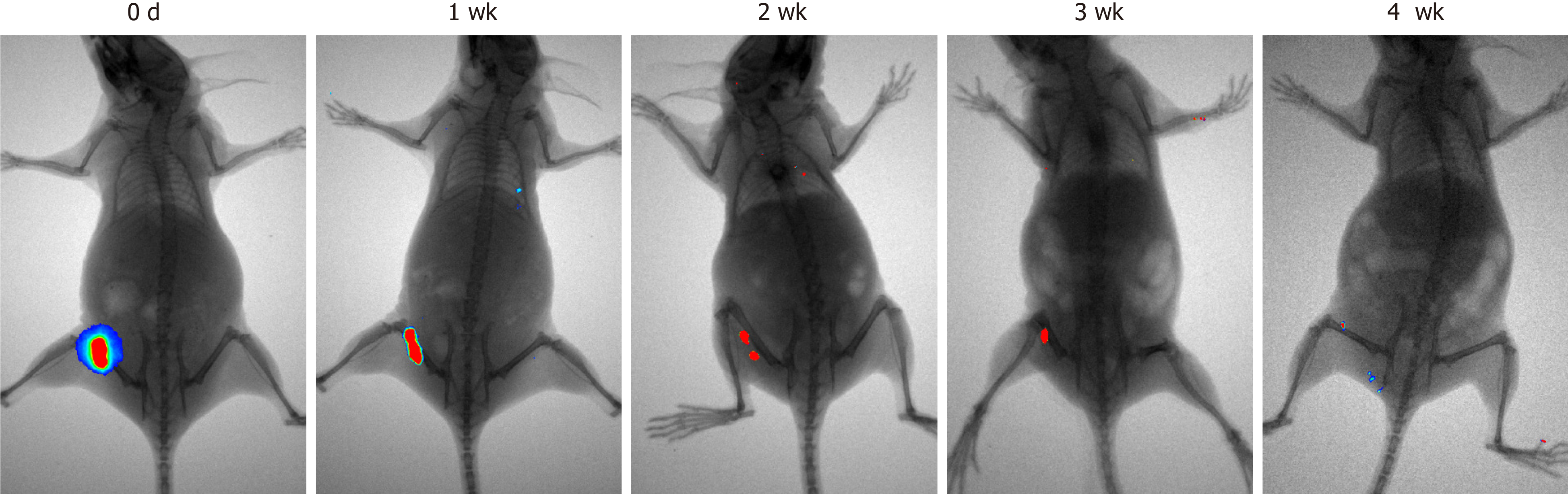
Figure 2 Tumorigenicity analysis of menstrual-derived stromal stem cells on nude mice 4 wk after subcutaneous injection.
Representative photographs show the cell proliferation of menstrual-derived stromal stem cells (MenSCs) on nude mice 4 wk after subcutaneous injection. Fluorescent expression, which represented the MenSCs, gradually decreased over time. No metastatic or proliferative fluorescent signals were detected in other parts of the body.
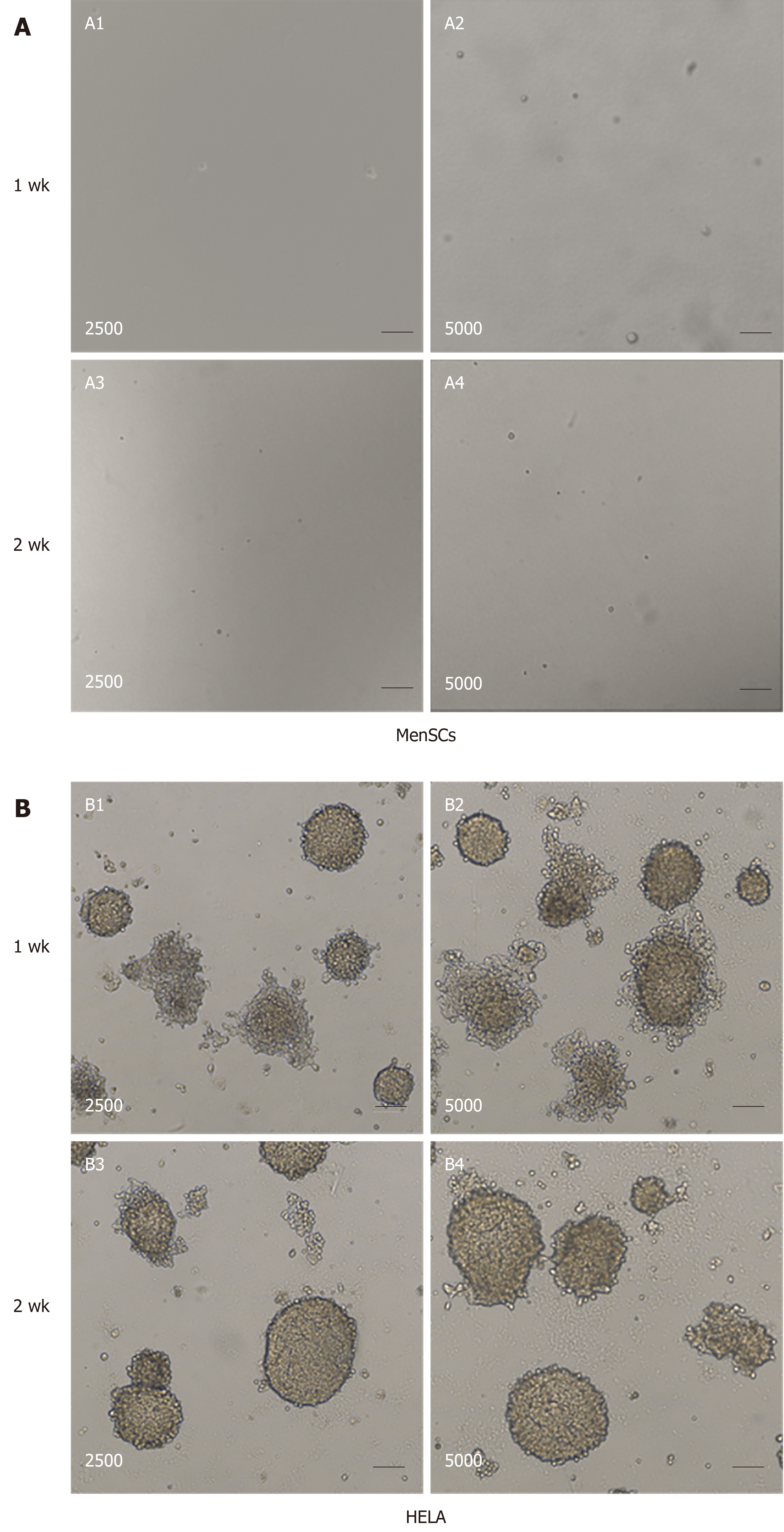
Figure 3 Tumorigenicity analyses of menstrual-derived stromal stem cells and Hela cells in soft-agar colony formation assay.
A: Representative photomicrographs show the cell malignant proliferation of 2500 and 5000 menstrual-derived stromal stem cells (MenSCs) after 1 and 2 wk of culturing. No cell colony was formed in the MenSCs group. B: Representative photomicrographs show the cell malignant proliferation of 2500 and 5000 HELA cells after 1 and 2 wk of culturing. Scale bar = 100 μm. MenSCs: Menstrual-derived stromal stem cells.
- Citation: Chang QY, Zhang SW, Li PP, Yuan ZW, Tan JC. Safety of menstrual blood-derived stromal cell transplantation in treatment of intrauterine adhesion. World J Stem Cells 2020; 12(5): 368-380
- URL: https://www.wjgnet.com/1948-0210/full/v12/i5/368.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i5.368