Copyright
©The Author(s) 2020.
World J Stem Cells. Nov 26, 2020; 12(11): 1255-1275
Published online Nov 26, 2020. doi: 10.4252/wjsc.v12.i11.1255
Published online Nov 26, 2020. doi: 10.4252/wjsc.v12.i11.1255
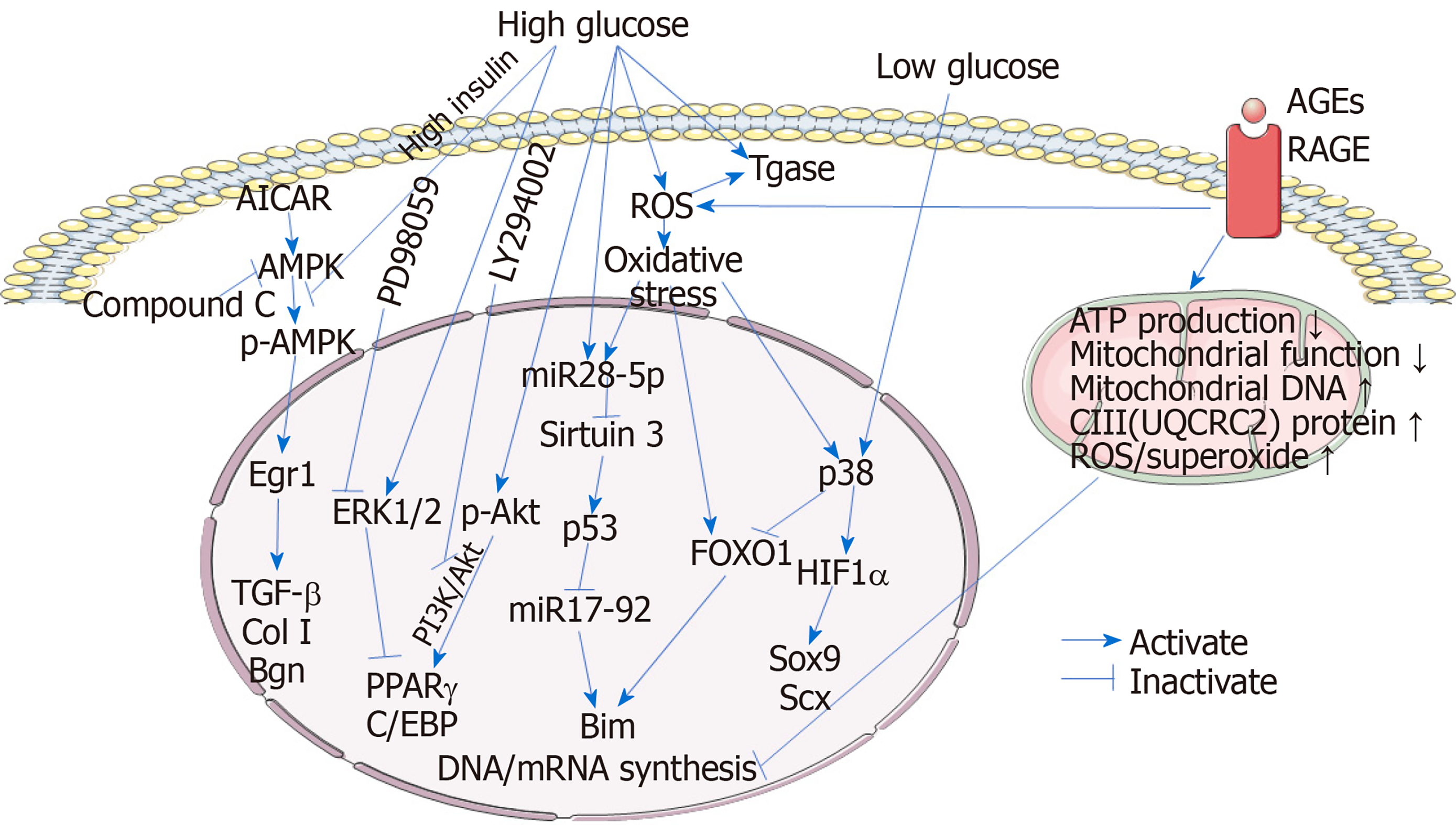
Figure 1 Mechanisms involved in the tenocytes during the process of diabetic tendinopathy.
High glucose and high insulin inhibit the expression of the tendon-related genes TGF-β, collagen I, and biglycan in tenocytes by inactivating the AMPK/Egr1 pathway. In addition, high glucose promotes the adipogenic transdifferentiation of tenocytes and increases the protein expression of p-Akt and p-ERK1/2. Activation of the PI3K/Akt pathway plays an essential role in maintaining the expression of the adipogenic transcription factors peroxisome proliferator-activated receptor γ (PPARγ) and C/EBP, however, activation of ERK signaling downregulates PPARγ expression, suggesting the fibroblast-to-adipocyte phenotypic transition induced by high glucose which can be inhibited by Akt inhibitor LY294002 and promoted by ERK inhibitor PD98059. High glucose stimulates transglutaminase (Tgase) activity, leading to an increase in extracellular matrix degradation. Reactive oxygen species (ROS) accumulation is also increased in tenocytes under high glucose conditions, triggering the oxidative stress and increasing Tgase activity. Oxidative stress increases both FOXO1 and hypoxia-inducible factor-1α (HIF1α) expression in tenocytes. Under high glucose conditions, miR28-5p is also upregulated, especially in oxidative stress-treated tenocytes. miR28-5p directly inhibits the expression of the p53 deacetylase sirtuin 3, resulting in an increase in acetylated p53. p53 inhibits the expression of miR17-92, repressing the degradation of the proapoptotic protein Bim. Meanwhile, FOXO1 promotes the transcription of Bim, the gene product of which is a proapoptotic protein. Inhibition of Bim degradation and upregulation of Bim transcription synergistically result in an increase in Bim, facilitating the tenocyte apoptosis. However, under low glucose conditions, miR28-5p-sirt3-p53 pathway is not stimulated. Instead, p38 MAPK is activated and acts on both FOXO1 and HIF1α, resulting in the inhibition of FOXO1 transcriptional activity and activation of HIF1α. HIF1α enhances the expression of the tendon-related genes Sox9 and Scx. Advanced glycation end-products (AGEs), which bind to receptor for AGEs, impair the mitochondrial functions, affecting the DNA and mRNA synthesis in tenocytes. In addition, mitochondrial and total ROS/superoxide production are also remarkably increased in AGEs-treated tenocytes. AGEs: Advanced glycation end-products; RAGE: Receptor for AGEs; ROS: Reactive oxygen species; Tgase: Transglutaminase; Col I: Collagen I; Bgn: Biglycan; PPARγ: Peroxisome proliferator-activated receptor γ; HIF1α: Hypoxia-inducible factor-1α.
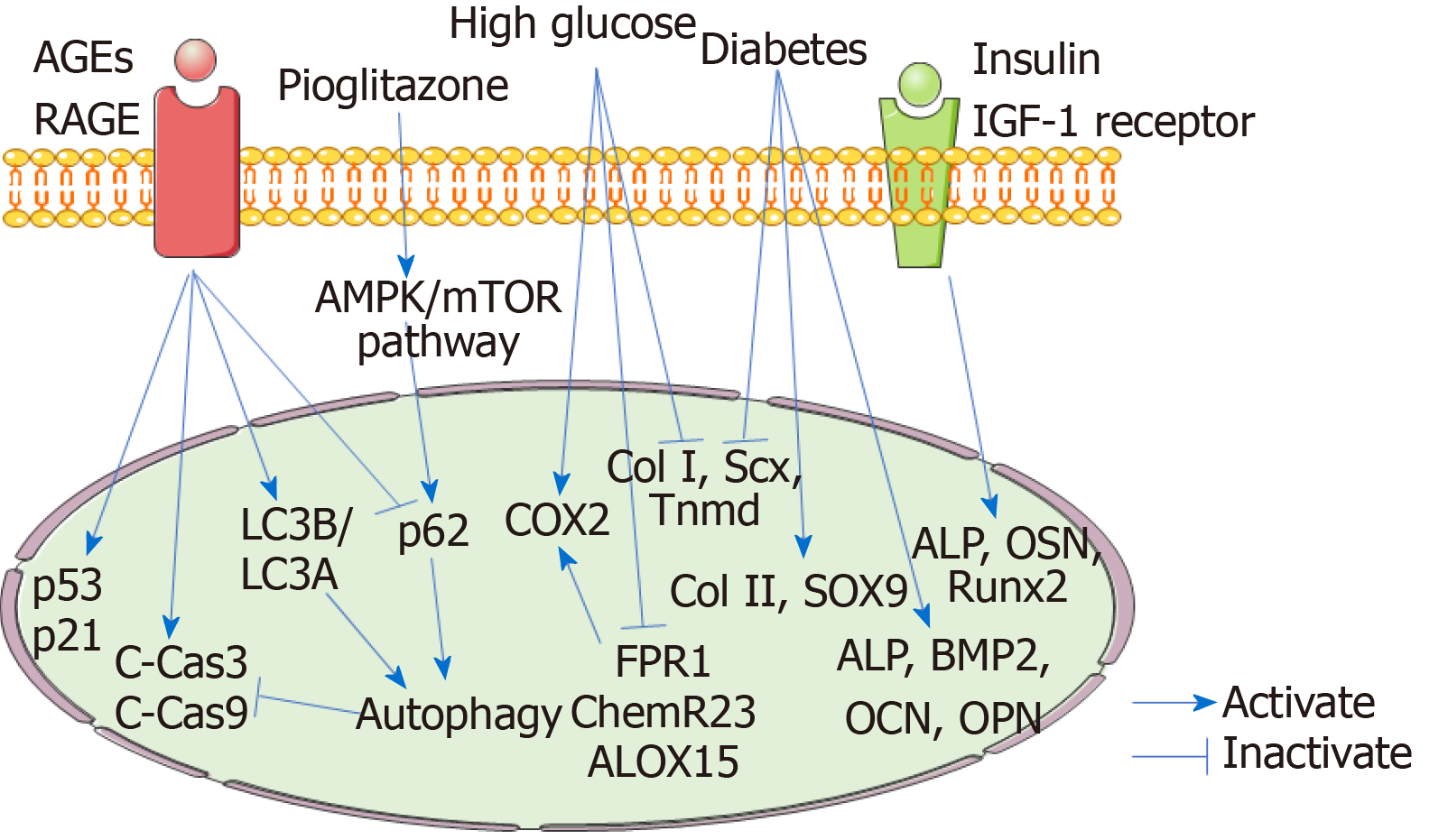
Figure 2 Mechanisms involved in the tendon stem/progenitor cells during the process of diabetic tendinopathy.
High glucose inhibits the expression of the tenogenic genes collagen I (Col I), scleraxis (Scx), and tenomodulin (Tnmd) in tendon stem/progenitor cells (TSPCs), indicating the suppressed tenogenic differentiation. Moreover, diabetic TSPCs exhibit increased osteogenic and chondrogenic differentiation abilities and decreased tenogenic differentiation ability with increased expression of osteochondrogenic markers alkaline phosphatase (ALP), bone morphogenetic protein 2, osteocalcin, osteopontin, Col II, and SOX9 and reduced expression of tenogenic genes Col I, Scx, and Tnmd. Insulin interacts with insulin-like growth factor-1 receptor to promote osteogenic differentiation of TSPCs by activating the expression of ALP, OSN, and runt-related transcription factor 2. These findings reveal that the erroneous differentiation of TSPCs plays important roles in the development and progression of diabetic tendinopathy. High glucose upregulates the expression of COX2 and downregulates the expression of FPR1, ChemR23, and ALOX15 in TSPCs, suggesting that high glucose stimulates an inflammatory response and weakens the pro-resolving responses, which results in persistent chronic inflammation in diabetic tendons. Advanced glycation end-products (AGEs) increase the expression of cleaved caspase-3 and cleaved caspase-9, suggesting an increase in TSPC apoptosis induced by AGEs. AGEs elevate the ratio of LC3B/LC3A and decrease P62 expression, indicating AGEs-induced autophagy. Autophagy plays protective roles against the AGE-induced apoptosis. AGEs also induced the TSPC senescence, with elevated expression of P53 and P21. Pioglitazone could decrease AGE-induced apoptosis by stimulating autophagy via activating the AMPK/mTOR pathway. AGEs: Advanced glycation end-products; RAGE: Receptor for AGEs; HIF1α: Hypoxia-inducible factor-1α; C-Cas3: Cleaved caspase-3; C-Cas9: Cleaved caspase-9; Col I: Collagen I; Scx: Scleraxis; Tnmd: Tenomodulin; ALP: Alkaline phosphatase; OSN: Osteonectin; Runx2: Runt-related transcription factor 2; BMP2: Bone morphogenetic protein 2; OPN: Osteopontin; OCN: Osteocalcin.
- Citation: Lu PP, Chen MH, Dai GC, Li YJ, Shi L, Rui YF. Understanding cellular and molecular mechanisms of pathogenesis of diabetic tendinopathy. World J Stem Cells 2020; 12(11): 1255-1275
- URL: https://www.wjgnet.com/1948-0210/full/v12/i11/1255.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i11.1255