Copyright
©The Author(s) 2018.
World J Stem Cells. Apr 26, 2018; 10(4): 34-42
Published online Apr 26, 2018. doi: 10.4252/wjsc.v10.i4.34
Published online Apr 26, 2018. doi: 10.4252/wjsc.v10.i4.34
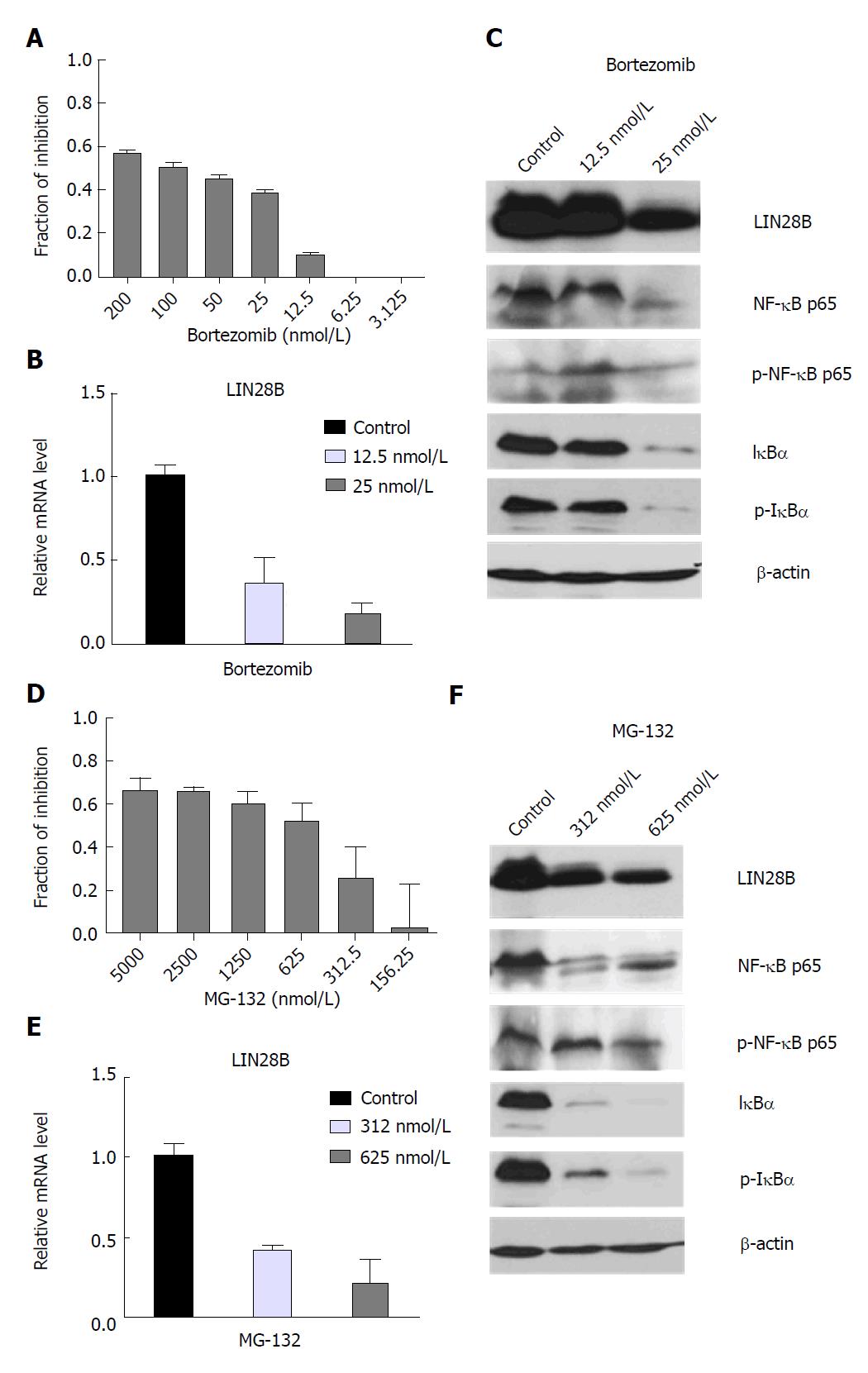
Figure 1 Effects of nuclear factor kappa B (NF-κB) inhibitors on acute myeloid leukemia cell viability and LIN28 mRNA and protein expression.
TF-1a cells were treated with DMSO control, different doses of Bortezomib as indicated, followed by CellTiter-Glo® Luminescent Cell Viability Assay (CTG assays) (A) or RNA extraction for qRT-PCR (B) and protein extraction for Western blot analysis (C), TF-1 cells were treated with DMSO control, different doses of MG-132 as indicated, followed by CTG assays (D) or RNA extraction for qRT-PCR (E) and protein extraction for Western blot analysis (F). For CTG assays, luminescence of each drug concentration and their controls were quantified. The relative inhibition induced by drug treatment was calculated relative to DMSO controls. For qRT-PCR analysis, the LIN28B mRNA level in DMSO control samples was set as 1 and the relative fold changes of LIN28B in drug treated samples were normalized to DMSO control samples. The experiments were triplicated (n = 3, mean ± SD). For Western blot analysis, β-actin was used as loading control.
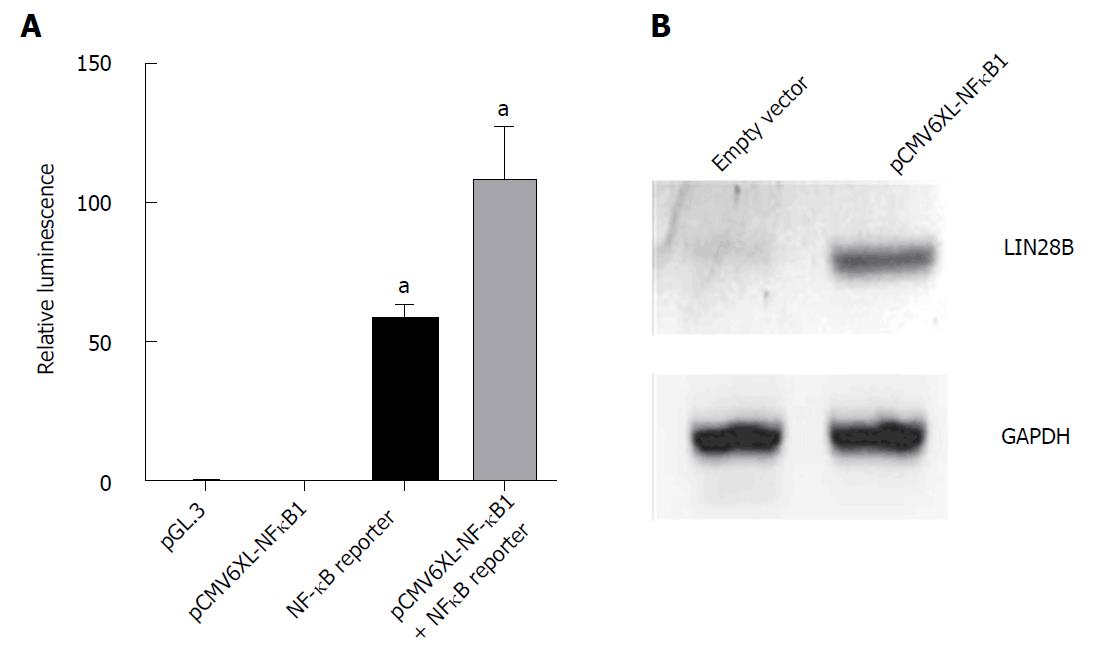
Figure 2 Overexpression of nuclear factor kappa B (NF-κB) in HEK293T cells.
A: HEK293T cells were co-transfected with 0.5 μg of pGL3.0, 2 μg of pCMV6-XL4 NF-κB1, 2 μg of NF-κB reporter gene and 10 ng of Renilla vector as indicated on the x-axis in 1mL of DMEM growth medium for 24 h prior to lysis for measuring the luminescence. All samples were normalized with renilla luciferase to ensure equal transfection efficiency. Cells transfected with PGL3 vector was used as negative control. The experiments were repeated 6 times (mean ± SD); B: Western blot analysis of LIN28B protein level in HEK293 cells receiving empty vector and NFκB1 vector as indicated. Proteins were extracted after 48 h of transfection. GAPDH was used as loading control.aP < 0.05.
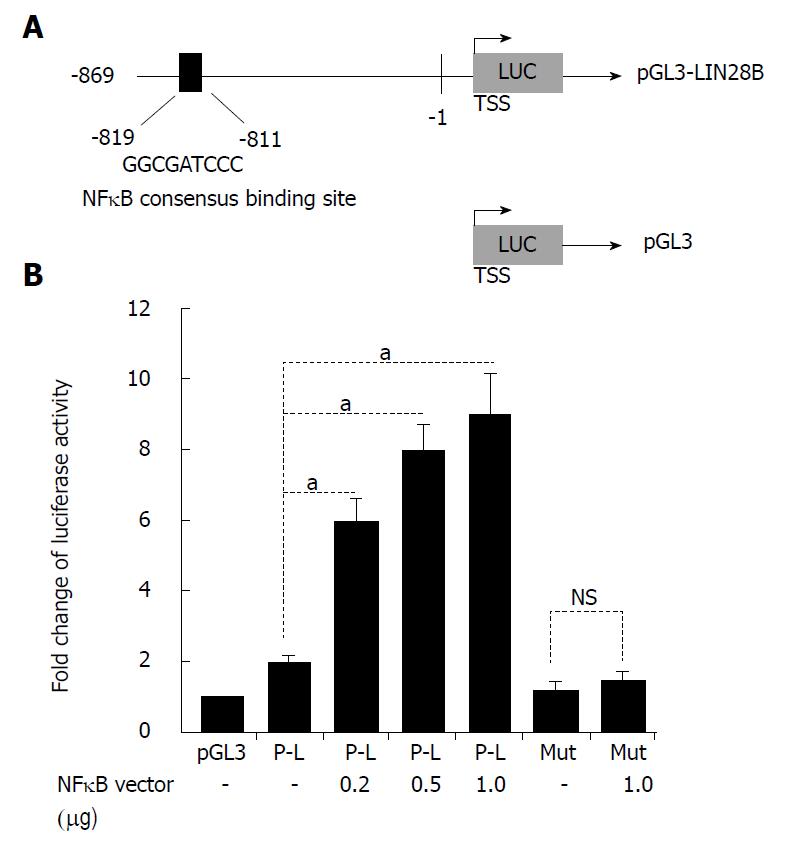
Figure 3 LIN28B is a target gene of nuclear factor kappa B (NF-κB).
A: Schematic representation of the human LIN28B promoter region cloned into pGL3 reporter constructs (pGL3-LIN28B). Black blocks show NF-κB-consensus-binding sites. The sequences and positions of the NF-κB-consensus-binding site shown; B: The pGL3-LIN28B reporter constructs (P-L), as well as the mutant construct, were introduced into 293T cells together with or without NF-κB p65 vector. After 24 h, the cells were subjected to dual-luciferase reporter assay, as described. The experiments were repeated 6 times. The baseline luciferase activity of pGL3 was set as 1 and the luciferase readings of all other samples were normalized with the baseline (n = 6, mean ± SD). TSS: Transcription start site. NS: Not significant. aP < 0.05
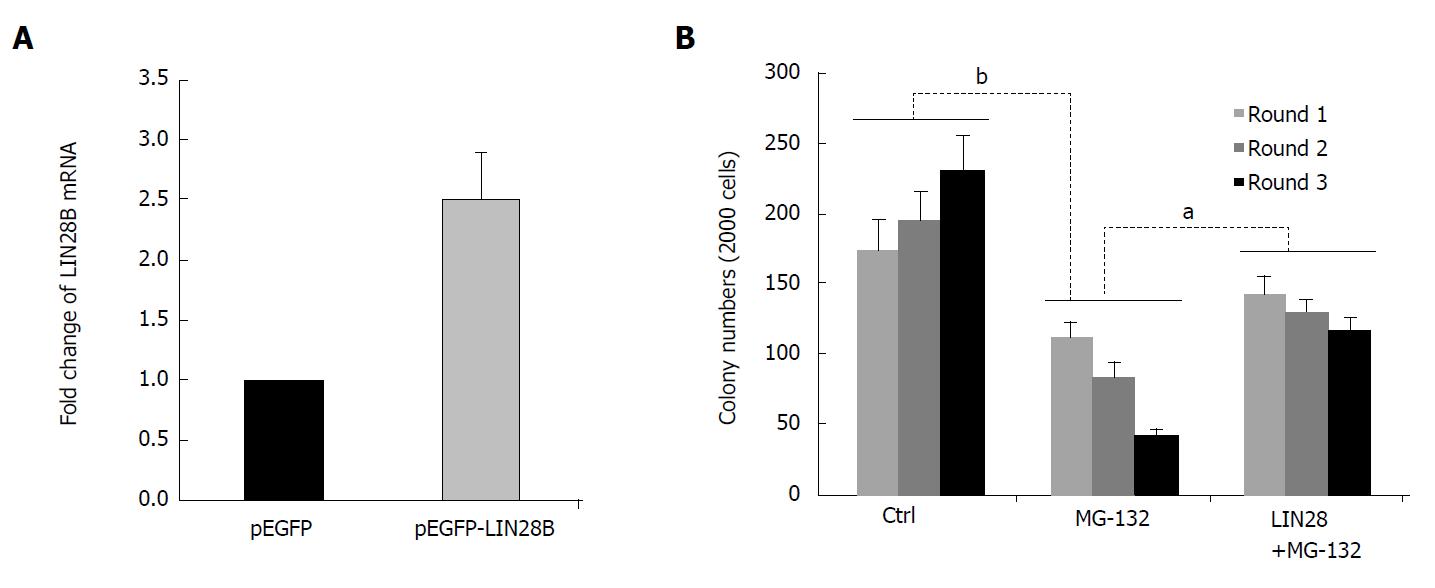
Figure 4 Role of LIN28B in nuclear factor kappa B (NF-B)-mediated leukemic stem cell-like properties.
A: RNAs of pEGFP and pEGFP-LIN28B transfected TF-1a cells, followed by qRT-PCR comparing LIN28B transcription level. The expression of LIN28B in each sample was normalized with GAPDH respectively (n = 3, mean ± SD); B: A total of 2000 cells from TF-1a or LIN28B overexpression cells were initially plated in methylcellulose medium containing either DMSO as control or MG-132 at 50 nmol/L, followed by another two rounds of serial replating assays. Quantification of colonies of indicated cells over 3 rounds of replating in methylcellulose medium. Bar figures represented 3 separated experiments (n = 3, mean ± SD). aP < 0.05; bP < 0.01.
- Citation: Zhou J, Chooi JY, Ching YQ, Quah JY, Toh SHM, Ng Y, Tan TZ, Chng WJ. NF-κB promotes the stem-like properties of leukemia cells by activation of LIN28B. World J Stem Cells 2018; 10(4): 34-42
- URL: https://www.wjgnet.com/1948-0210/full/v10/i4/34.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i4.34