修回日期: 2024-08-03
接受日期: 2024-08-14
在线出版日期: 2024-08-28
胃癌是常见的消化道肿瘤, 具有较高的发病率和死亡率. 环状RNAs(circular RNAs, circRNAs)被认为是多种人类癌症进展的关键调控子, 包括胃癌. 研究显示circRASSF2作为促癌因子调控癌症进程. 然而其在胃癌进展中的作用及潜在分子机制尚不清楚.
探讨circRASSF2影响胃癌细胞增殖、迁移和凋亡的分子机制.
用实时荧光定量PCR分析胃癌组织、癌旁组织及GES-1、HGC-27和AGS细胞中circRASSF2和miR-218-5p的表达; 双荧光素酶报告实验分析靶向关系; 将HGC-27和AGS细胞分为si-circRASSF2组、si-NC组、miR-218-5p mimic组、miR-NC组、si-circRASSF2+anti-miR-NC组、si-circRASSF2+miR-218-5p Inhibitor组; 3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐检测细胞抑制率; 克隆形成实验检测克隆形成数; 流式细胞术检测细胞凋亡率; Transwell检测细胞迁移数.
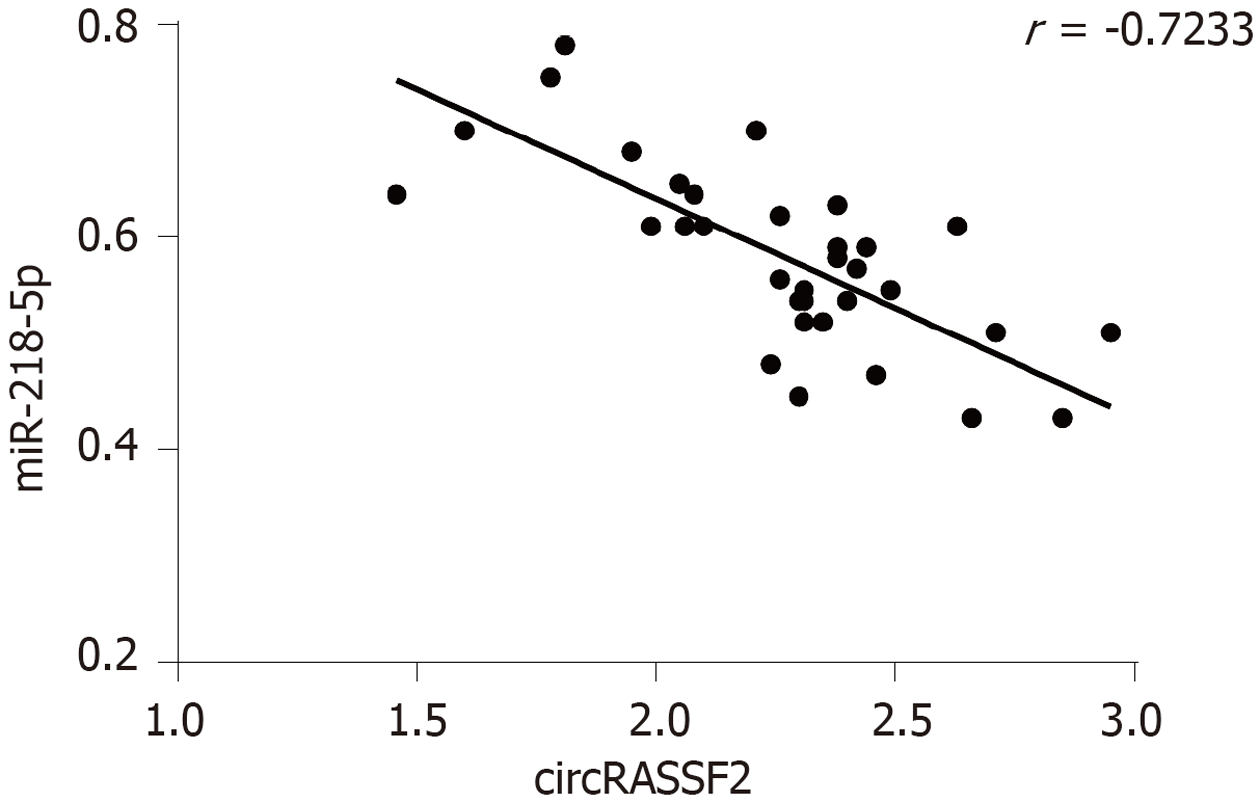
胃癌组织中circRASSF2表达上升, miR-218-5p表达下降, 且二者表达呈负相关(P<0.05); 在胃癌HGC-27和AGS细胞中, circRASSF2表达上调, 而miR-218-5p表达下调(P<0.05). circRASSF2靶向调控miR-218-5p; 敲低circRASSF2或过表达miR-218-5p促进HGC-27和AGS细胞抑制率和凋亡率, 而降低克隆形成数和迁移细胞数(P<0.05). 下调miR-218-5p可逆转circRASSF2敲低对细胞增殖、迁移、凋亡的影响.
circRASSF2通过靶向miR-218-5p加速胃癌细胞增殖、迁移、抑制凋亡.
核心提要: CircRASSF2在胃癌组织和细胞中上调而miR-218-5p下调. circRASSF2与miR-218-5p存在互补结合位点, 且circRASSF2可以靶向miR-218-5p促进胃癌细胞增殖和迁移, 加速胃癌恶性进展.
引文著录: 骆超, 朱迪, 陈跃华. CircRASSF2靶向miR-218-5p调控胃癌细胞增殖、迁移和凋亡的研究. 世界华人消化杂志 2024; 32(8): 608-615
Revised: August 3, 2024
Accepted: August 14, 2024
Published online: August 28, 2024
Gastric cancer is a common digestive tract tumor with high morbidity and mortality. Circular RNAs (circRNAs) are considered to be key regulators of the progression of a variety of human cancers, including gastric cancer. Studies have shown that circRASSF2 acts as an oncogene to regulate cancer progression. However, its role and underlying molecular mechanisms in gastric cancer progression are still unclear.
To explore the molecular mechanism by which circRASSF2 regulates gastric cancer cell proliferation, migration, and apoptosis.
The expression of circRASSF2 and miR-218-5p in gastric cancer tissues, adjacent tissues, and GES-1, HGC-27 and AGS cells was examined by real-time quantitative PCR. Dual luciferase reporter assay was used to assess the targeting relationship of circRASSF2 and miR-218-5p. HGC-27 and AGS cells were divided into the following groups: si-circRASSF2 group, si-NC group, miR-218-5p mimic group, miR-NC group, si-circRASSF2 + anti-miR-NC group, and si-circRASSF2 + miR-218-5p inhibitor group. MTT assay and clone formation assay were used to detect cell proliferation. Flow cytometry was used to detect cell apoptosis. Transwell assay was used to detect cell migration.
CircRASSF2 expression increased and miR-218-5p expression decreased in gastric cancer tissues, and their expression was negatively correlated (P < 0.05); circRASSF2 was upregulated and miR-218-5p was downregulated in gastric cancer HGC-27 and AGS cells (P < 0.05). Dual luciferase reporter assay showed that circRASSF2 targets miR-218-5p. CircRASSF2 knockdown or miR-218-5p overexpression increased HGC-27 and AGS cell poptosis, while reduced cell proliferation and migration (P < 0.05). Down-regulation of miR-218-5p reversed the effect of circRASSF2 knockdown on cell proliferation, migration, and apoptosis.
CircRASSF2 promotes proliferation and migration and suppresses apoptosis of gastric cancer cells by targeting miR-218-5p.
- Citation: Luo C, Zhu D, Chen YH. CircRASSF2 targets miR-218-5p to regulate gastric cancer cell proliferation, migration, and apoptosis. Shijie Huaren Xiaohua Zazhi 2024; 32(8): 608-615
- URL: https://www.wjgnet.com/1009-3079/full/v32/i8/608.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v32.i8.608
胃癌是世界上最常见的恶性肿瘤之一, 分子靶向治疗展现出巨大的潜力, 而其主要挑战是耐药性, 因此需要探究更多的分子靶点以研发靶向药物[1,2]. 非编码RNA(non-coding RNA, ncRNA)是功能性RNA分子, 其失调与胃癌的进展有关. 微小RNAs(microRNAs, miRNAs)、环状RNAs(circular RNAs, circRNAs)具有作为胃癌生物标志物或治疗靶点的潜力[3,4]. 研究报道[5]miR-218-5p可以抑制胃癌细胞的转移和上皮-间质转化. 过表达miR-218-5p能够抑制胃癌细胞的增殖并诱导细胞周期阻滞[6]. 此外, miR-218-5p可能靶向锌指蛋白114抑制胃癌细胞转移[7]. 以上研究表明miR-218-5p参与胃癌进展, 且受circRNAs调控. 经过软件预测, miR-218-5p可以与circRASSF2结合. circRASSF2在结直肠癌组织中显著上调, 敲低circRASSF2通过miR-195-5p/卷曲蛋白4轴抑制结直肠癌细胞增殖和转移[8]. 在喉鳞状细胞癌中, circRASSF2在癌组织中表达升高, 其敲低可通过miR-302b-3p/胰岛素样生长因子1受体显著抑制体外细胞增殖和迁移[9]. 然而circRASSF2在胃癌进展中的作用暂不清楚. 本实验探究是否circRASSF2靶向miR-218-5p调控胃癌进展.
于本院收集35例胃癌患者的癌组织及癌旁组织(表1), 所有样品储存在-80 ℃. 所有患者均知情且同意. 本研究获得了本院伦理委员会的批准.
| 临床参数 | n | |
| 年龄 | <60 | 17 |
| ≥60 | 18 | |
| 性别 | Male | 19 |
| Female | 16 | |
| 临床分期 | Ⅰ-Ⅱ | 20 |
| Ⅲ-Ⅳ | 15 | |
| 肿瘤大小 | <5 cm | 23 |
| ≥5 cm | 12 | |
细胞与主要试剂: 胃黏膜上皮细胞GES-1、胃癌细胞HGC-27、AGS(美国ATCC); RPMI-1640培养基(美国Gibco); Trizol、cDNA合成试剂盒、荧光定量PCR试剂盒(日本Takara); 双荧光素酶检测试剂盒、MTT试剂盒、凋亡检测试剂盒(上海碧云天); Transwell小室(美国Corning); Lipofectamine 3000(美国Invitrogen); si-circRASSF2、si-NC、miR-218-5p mimic、miR-NC、anti-miR-NC、miR-218-5p Inhibitor(广州锐博).
1.2.1 细胞处理与分组: 用RPMI-1640培养基培养GES-1、HGC-27、AGS细胞; 根据Lipofectamine 3000说明书, 将si-circRASSF2 a、b、c及si-NC转染至HGC-27和AGS细胞, 用RT-qPCR检测circRASSF2的表达水平, 确定转染效率. 使用Lipofectamine 3000将si-circRASSF2、si-NC、miR-218-5p mimic、miR-NC、si-circRASSF2+anti-miR-NC、si-circRASSF2+miR-218-5p Inhibitor分别转染至HGC-27和AGS细胞, 分为si-circRASSF2组、si-NC组、miR-218-5p mimic组、miR-NC组、si-circRASSF2+anti-miR-NC组、si-circRASSF2+miR-218-5p Inhibitor组.
1.2.2 实时荧光定量PCR(real-time quantitative PCR, RT-qPCR): 利用Trizol从组织(100 mg胃癌组织或癌旁组织置1.5 mL的EP管中, 加入1 mL的Trizol充分匀浆)和细胞(5×107个细胞置1.5 mL的EP管中, 加入1 mL的Trizol混匀)中提取总RNA, 随后利用cDNA合成试剂盒合成cDNA. 最后, 利用荧光定量PCR试剂盒在ABI StepOnePlus荧光定量PCR仪进行PCR扩增, 反应条件如下: 95 ℃ 2 min, 95 ℃ 30 s, 60 ℃ 30 s, 72 ℃ 30 s(循环40次). 用2-△△Ct法计算相对表达, 以GAPDH或U6为内参. 引物序列如下: circRASSF2, F 5'-CTTTTCAAAGAGAGTGGCCCAG-3', R 5'-ACGGTGTACTTGCGCATCAG-3'; GAPDH, F 5'-TCGGAGTCAACGGATTTGGT-3', R 5'-TTCCCGTTCTCAGCCTTGAC-3'; miR-218-5p, F 5'-AACACGAACTAGATTGGTACA-3', R 5'-AGTCTCAGGGTCCGAGGTATTC-3'; U6, F 5'-ATTGGAACGATACAGAGAAGATT-3', R 5'-GGAACGCTTCACGAATTTG-3'.
1.2.3 双荧光素酶报告实验: 利用starbase软件预 circRASSF2和miR-218-5p的结合位点, 并根据其互补序列设计circRASSF2野生型(wild-type, wt)/突变型(mutant-type, mut)序列. 随后, 将含有wt/mut的circRASSF2序列克隆到psi-CHECK2荧光素酶载体中, 合成wt-circRASSF2/mut-circRASSF2载体. 利用Lipofectamine 3000将miR-NC/miR-218-5p和wt-circRASSF2/mut-circRASSF2载体分贝转染入HGC-27和AGS细胞. 48 h后, 利用双荧光素酶试剂盒分析细胞相对荧光素酶活性.
1.2.4 3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, MTT): HGC-27和AGS细胞接种到96孔板培养48 h. 随后, 细胞与MTT溶液孵育4 h, 然后与DMSO孵育10 min. 最后, 使用酶标仪检测波长在490 nm的光密度值(optical density, OD), 评估细胞抑制率.
1.2.5 克隆形成实验: HGC-27和AGS细胞接种于6孔板中, 每2 d更换培养基, 共培养14 d. 随后, 形成的细胞克隆经多聚甲醛固定和结晶紫染色, 在显微镜下计数细胞克隆数.
1.2.6 流式细胞术: 收集的HGC-27和AGS细胞用PBS清洗后, 用结合缓冲液重悬. 细胞悬浮液与Annexin V-FITC和PI孵育15 min, 最后用流式细胞仪分析细胞凋亡率.
1.2.7 Transwell: 用无血清培养基重悬的HGC-27和AGS细胞添加到Transwell上室, 下室添加完全培养基. 培养48 h后收集下表面的细胞, 经过多聚甲醛固定和结晶紫染色, 显微镜拍摄并计数迁移细胞数.
统计学处理 采用SPSS 20.0软件进行统计学分析, 计量资料用mean±SD表示, 组间比较行t检验或单因素方差分析. 以P<0.05为差异有统计学意义.
与癌旁组织对比, 胃癌组织中circRASSF2上调, miR-218-5p下调, 二者表达呈负相关(P<0.05)(图1, 表2). 与GES-1相比, 胃癌HGC-27和AGS细胞中circRASSF2表达上升, 而miR-218-5p表达下降(P<0.05)(表3).
Starbase预测circRASSF2和miR-218-5p的结合位点(图2). 通过双荧光素酶分析, 我们确定miR-218-5p可以下调wt-circRASSF2载体的荧光素酶活性(P<0.05), 而不影响mut-circRASSF2载体的荧光素酶活性(表4). 此外, si-circRASSF2可以上调miR-218-5p表达(P<0.05)(表5).
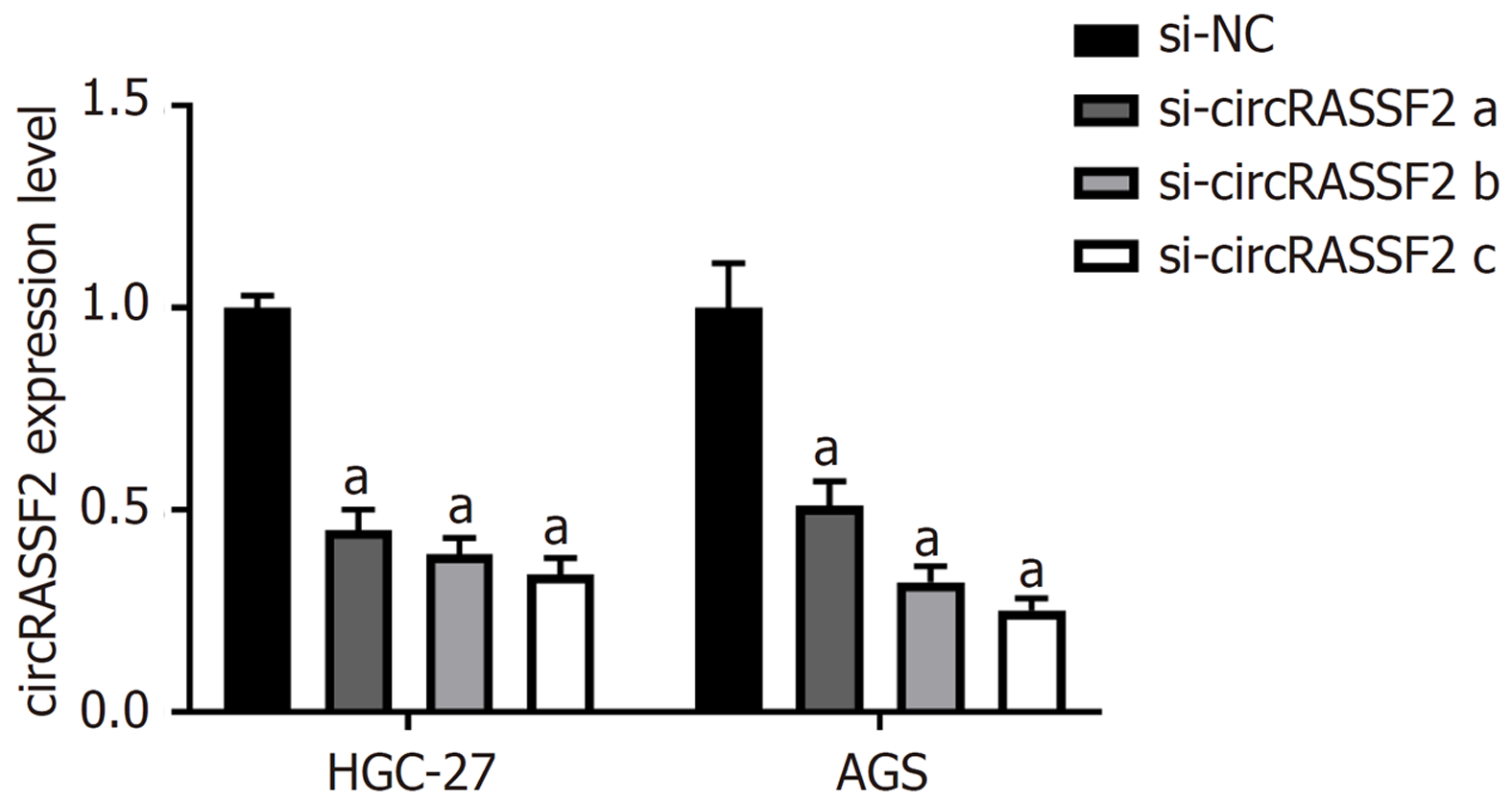
与si-NC组相比, 转染si-circRASSF2 a、b、c后circRASSF2表达降低(P<0.05), 表明转染成功(图3).
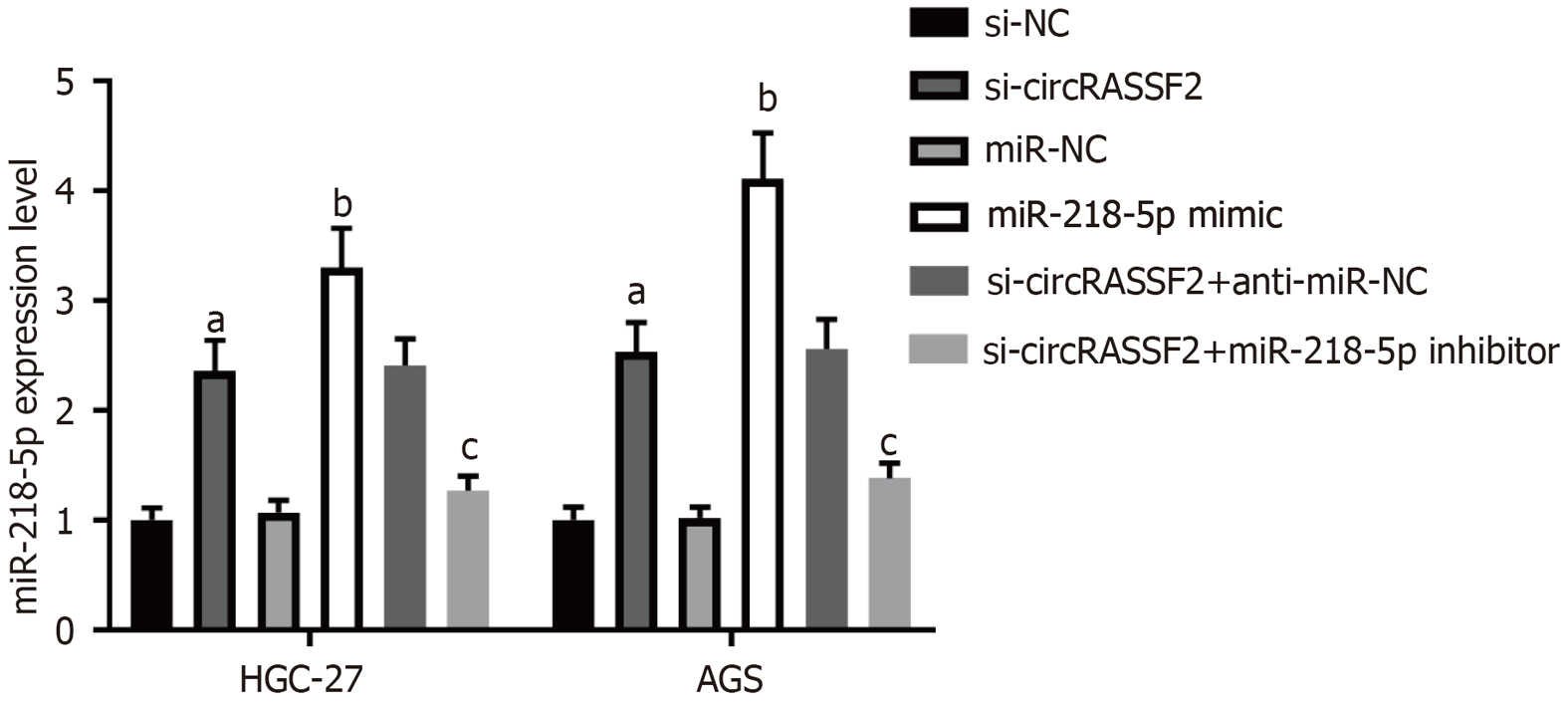
在si-circRASSF2组和miR-218-5p mimic组中, HGC-27和AGS细胞中miR-218-5p表达上调, 而共转染si-circRASSF2+miR-218-5p Inhibitor后, miR-218-5p表达下调, 图4.
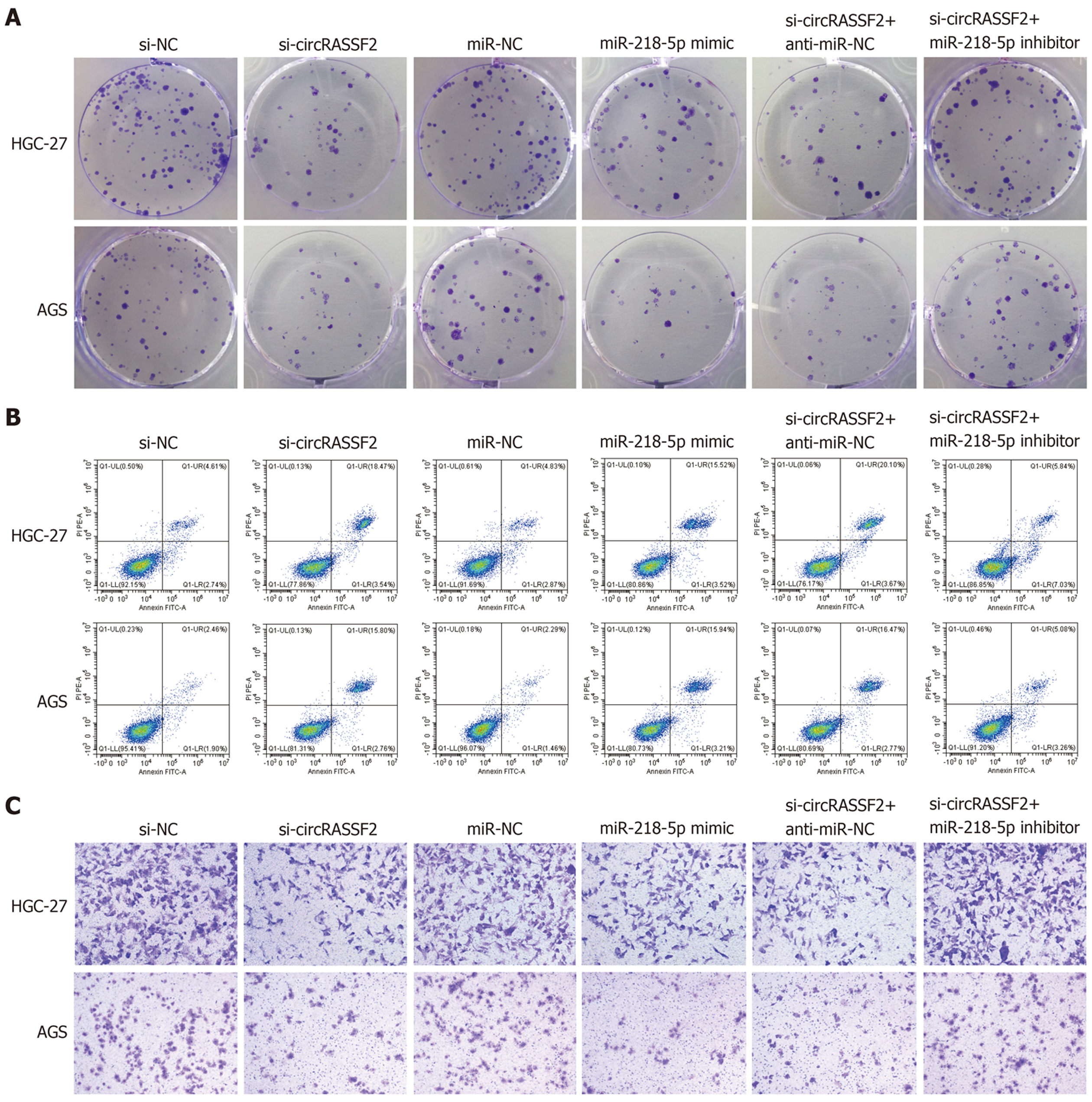
在si-circRASSF2组和miR-218-5p mimic组中, HGC-27和AGS细胞抑制率和凋亡率上调, 而克隆形成数和迁移数下降(P<0.05); 共转染si-circRASSF2+miR-218-5p Inhibitor后, HGC-27和AGS细胞抑制率和凋亡率下调, 而克隆形成数和迁移数上升(P<0.05)(图5, 表6).
| 分组 | 抑制率/% | 克隆形成数/个 | 凋亡率/% | 迁移细胞数/个 | |
| HGC-27 | si-NC | 0.00±0.00 | 112.52±6.25 | 8.07±0.47 | 169.89±10.80 |
| si-circRASSF2 | 55.23±2.34a | 40.27±3.36a | 22.89±1.15a | 73.00±2.54a | |
| miR-NC | 0.03±0.03 | 101.40±9.33 | 8.11±0.57 | 171.00±11.17 | |
| miR-218-5p mimic | 44.19±2.70b | 42.26±4.11b | 19.78±1.13b | 85.78±3.91b | |
| si-circRASSF2+anti-miR-NC | 55.01±2.75 | 37.21±3.38 | 23.06±1.36 | 69.56±3.47 | |
| si-circRASSF2+miR-218-5p Inhibitor | 20.36±1.22c | 89.27±8.25c | 13.31±0.79c | 146.44±7.26c | |
| AGS | si-NC | 0.00±0.00 | 55.26±6.42 | 5.64±0.67 | 116.51±12.14 |
| si-circRASSF2 | 43.84±5.77a | 24.33±2.27a | 18.96±1.52a | 56.58±6.36a | |
| miR-NC | 0.01±0.02 | 46.28±4.18 | 4.98±0.58 | 113.84±12.21 | |
| miR-218-5p mimic | 45.26±5.10b | 18.22±2.14b | 19.27±2.21b | 52.27±5.95b | |
| si-circRASSF2+anti-miR-NC | 44.1±4.5 | 19.33±1.74 | 19.04±2.02 | 57.43±6.27 | |
| si-circRASSF2+miR-218-5p Inhibitor | 18.62±1.92c | 40.39±3.85c | 9.25±0.97c | 101.22±10.61c | |
| F | 1569.442 | 375.574 | 429.625 | 379.610 | |
| P | <0.05 | <0.05 | <0.05 | <0.05 |
新的分子靶向药物是改善胃癌治疗效果的希望[10,11]. 既往的研究显示[12,13]miR-218-5p作为抑癌因子调控多种癌症进程. 在膀胱癌中, miR-218-5p表达下调, 且过表达miR-218-5p可抑制膀胱癌细胞的增殖和转移, 促进细胞凋亡[14]. MiR-218-5p在胶质瘤组织中显著低表达, 其表达下调与脑胶质瘤患者不良预后相关, 过表达miR-218-5p可抑制胶质瘤细胞的增殖、迁移和侵袭并促进其凋亡[15]. 尽管既往的研究证实了miR-218-5p在胃癌进展中的消极作用[5-7], 但是miR-218-5p相关的分子轴仍需要进一步揭示. 本结果显示, miR-218-5p在胃癌组织中和细胞中下调, 且过表达miR-218-5p促进细胞抑制率和凋亡率, 而降低克隆形成数和迁移细胞数. 表明miR-218-5p可抑制胃癌细胞增殖、迁移、提高凋亡, 这与既往的研究结果一致[5-7]. 这些数据为miR-218-5p成为胃癌治疗的潜在分子靶点提供了新的证据.
由于具有稳定的表达, circRNA被认为是人类疾病的潜在分子治疗靶点和诊断指标[16,17]. 越来越多的研究显示circRNA是胃癌进展的关键调控子, 其异常表达与胃癌进展密切相关[18,19]. 例如: circ_0136666在胃癌组织和细胞中广泛且高表达, 其可以促进胃癌肿瘤增殖和肿瘤微环境形成, 导致肿瘤发生免疫逃逸[20]. 此外, circLDLR的过表达增强了胃癌细胞的增殖和有氧糖酵解, 并阻碍了细胞凋亡, 进而加速胃癌恶性进展[21]. 以上证据证实了circRNA在胃癌进展中的具有重要作用. CircRASSF2被证实作为促癌因子参与调控多种癌症进展. Yang等[8]的研究显示circRASSF2在结直肠癌组织和细胞中表达上调, 其高表达与结直肠癌患者的不良预后相关, 且敲低circRASSF2可抑制结直肠癌细胞的增殖、迁移、侵袭并促进其凋亡. 此外, circRASSF2在乳腺癌组织和血清中的表达显著增加, 与远处转移、淋巴结转移、TNM分期、分化和肿瘤大小呈正相关[22]. 然而, circRASSF2在胃癌进展中的作用尚不清楚. 本实验经过软件预测, 发现miR-218-5p与circRASSF2之间存在结合位点, 并通过双荧光素酶进一步证实了二者的互作. 此外, 我们的结果显示, circRASSF2在胃癌组织和细胞中上调, 沉默circRASSF2增加细胞抑制率和凋亡率, 而下降克隆形成数和迁移细胞数, 表明circRASSF2促进胃癌细胞增殖、迁移、抑制凋亡. 重要的是, 下调miR-218-5p可减弱沉默circRASSF2对胃癌细胞增殖、迁移和凋亡的影响, 进一步表明circRASSF2通过靶向miR-218-5p促进胃癌恶性进展.
综上所述, 本研究结果显示, circRASSF2通过靶向miR-218-5p促进胃癌细胞增殖、迁移、抑制凋亡. CircRASSF2/miR-218-5p分子轴的提出为胃癌的治疗提供了潜在分子靶点.
胃癌是我国最常见的恶性肿瘤之一, 在我国其发病率居各类肿瘤的首位, 对患者生命造成了巨大威胁. 因此, 有必要阐明影响其进展的分子机制,为其治疗靶点的开发提供新思路.
探究影响胃癌进展的分子机制可能为其治疗提供潜在分子靶点.
揭示circRASSF2是否通过调控miR-218-5p调控胃癌细胞增殖、迁移和凋亡.
通过转染si-circRASSF2分析circRASSF2敲低对胃癌细胞增殖、迁移和凋亡的影响; 通过转染miR-218-5p mimic确定miR-218-5p过表达对胃癌细胞增殖、迁移和凋亡的影响; 双荧光素酶报告实验确定circRASSF2和miR-218-5p的靶向关系; 通过共转染si-circRASSF2和miR-218-5p Inhibitor确定circRASSF2是否通过调控miR-218-5p介导胃癌细胞增殖、迁移和凋亡.
CircRASSF2敲低或miR-218-5p过表达均可以抑制胃癌细胞增殖、迁移、促进凋亡; CircRASSF2可以靶向miR-218-5p, 且miR-218-5p Inhibitor可以逆转LncRNA si-circRASSF2对胃癌细胞增殖、迁移和凋亡的作用.
CircRASSF2通过靶向miR-218-5p促进胃癌细胞增殖、迁移、抑制凋亡, 这可能为胃癌的治疗提供潜在分子靶点.
未来仍需要探究胃癌的发病机制, 为胃癌的临床治疗提供新思路.
学科分类: 胃肠病学和肝病学
手稿来源地: 浙江省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): 0
D级 (一般): D, D
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [PubMed] [DOI] |
| 2. | Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. 2022;86:566-582. [PubMed] [DOI] |
| 3. | Chen L, Deng J. Role of non-coding RNA in immune microen-vironment and anticancer therapy of gastric cancer. J Mol Med (Berl). 2022;100:1703-1719. [PubMed] [DOI] |
| 4. | Wang ZX, Zhang GJ, Yang XF, Feng SJ, Ji SS, Qi YB. miRNA-633 and KAI1 as Potential Biomarkers of Malignant Melanoma with Gastric Cancer. Comb Chem High Throughput Screen. 2023;26:1001-1014. [PubMed] [DOI] |
| 5. | Zhang T, Beeharry MK, Wang Z, Zhu Z, Li J, Li C. YY1-modulated long non-coding RNA SNHG12 promotes gastric cancer metastasis by activating the miR-218-5p/YWHAZ axis. Int J Biol Sci. 2021;17:1629-1643. [PubMed] [DOI] |
| 6. | Deng M, Zeng C, Lu X, He X, Zhang R, Qiu Q, Zheng G, Jia X, Liu H, He Z. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403:175-185. [PubMed] [DOI] |
| 7. | Feng Z, Li L, Zeng Q, Zhang Y, Tu Y, Chen W, Shu X, Wu A, Xiong J, Cao Y, Li Z. RNF114 Silencing Inhibits the Proliferation and Metastasis of Gastric Cancer. J Cancer. 2022;13:565-578. [PubMed] [DOI] |
| 8. | Yang L, Bi T, Zhou S, Lan Y, Zhang R. CircRASSF2 facilitates the proliferation and metastasis of colorectal cancer by mediating the activity of Wnt/β-catenin signaling pathway by regulating the miR-195-5p/FZD4 axis. Anticancer Drugs. 2021;32:919-929. [PubMed] [DOI] |
| 9. | Tian L, Cao J, Jiao H, Zhang J, Ren X, Liu X, Liu M, Sun Y. CircRASSF2 promotes laryngeal squamous cell carcinoma progression by regulating the miR-302b-3p/IGF-1R axis. Clin Sci (Lond). 2019;133:1053-1066. [PubMed] [DOI] |
| 10. | Wu S, Xu P, Zhang F. Advances in targeted therapy for gastric cancer based on tumor driver genes. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023;53:73-83. [PubMed] [DOI] |
| 11. | Chen Y, Bai B, Ying K, Pan H, Xie B. Anti-PD-1 combined with targeted therapy: Theory and practice in gastric and colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188775. [PubMed] [DOI] |
| 12. | Naso FD, Bruqi K, Manzini V, Chiurchiù V, D'Onofrio M, Arisi I, Strappazzon F. miR-218-5p and doxorubicin combination enhances anticancer activity in breast cancer cells through Parkin-dependent mitophagy inhibition. Cell Death Discov. 2024;10:149. [PubMed] [DOI] |
| 13. | Pan F, Zhang J, Tang B, Jing L, Qiu B, Zha Z. The novel circ_0028171/miR-218-5p/IKBKB axis promotes osteosarcoma cancer progression. Cancer Cell Int. 2020;20:484. [PubMed] [DOI] |
| 14. | Li Y, Shi B, Dong F, Zhu X, Liu B, Liu Y. LncRNA KCNQ1OT1 facilitates the progression of bladder cancer by targeting MiR-218-5p/HS3ST3B1. Cancer Gene Ther. 2021;28:212-220. [PubMed] [DOI] |
| 15. | Gu J, Ge X, You A, Li J, Zhang Y, Rao G, Wang J, Zhang K, Liu X, Wu X, Cheng L, Zhu M, Wang D. miR-218-5p inhibits the malignant progression of glioma via targeting TCF12. Tumori. 2022;108:338-346. [PubMed] [DOI] |
| 16. | Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188-206. [PubMed] [DOI] |
| 17. | Zhang Y, Luo J, Yang W, Ye WC. CircRNAs in colorectal cancer: potential biomarkers and therapeutic targets. Cell Death Dis. 2023;14:353. [PubMed] [DOI] |
| 18. | Zheng Y, Li Z, Wang Y, Chen W, Lin Y, Guo J, Ye G. CircRNA: A new class of targets for gastric cancer drug resistance therapy. Pathol Oncol Res. 2023;29:1611033. [PubMed] [DOI] |
| 19. | Hossain MT, Li S, Reza MS, Feng S, Zhang X, Jin Z, Wei Y, Peng Y. Identification of circRNA Biomarker for Gastric Cancer through Integrated Analysis. Front Mol Biosci. 2022;9:857320. [PubMed] [DOI] |
| 20. | Miao Z, Li J, Wang Y, Shi M, Gu X, Zhang X, Wei F, Tang X, Zheng L, Xing Y. Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation. Mol Cancer. 2023;22:205. [PubMed] [DOI] |
| 21. | Zeng F, Zhao J, Tong M, He W, Li N, Fan Y, Zhu Y, Zhang L, Zhang H. CircRNA LDLR promotes proliferation and aerobic glycolysis of gastric cancer cells by targeting CHD1 with miR-449b-5p. Turk J Biol. 2024;48:46-58. [PubMed] [DOI] |
| 22. | Zhong W, Bao L, Yuan Y, Meng Y. CircRASSF2 acts as a prognostic factor and promotes breast cancer progression by modulating miR-1205/HOXA1 axis. Bioengineered. 2021;12:3014-3028. [PubMed] [DOI] |