修回日期: 2023-06-10
接受日期: 2023-06-29
在线出版日期: 2023-07-08
结肠癌是临床上常见的一种恶性肿瘤, 有研究报道, 环状RNA(circular RNA, circRNA)参与结肠癌的发生发展. 其中, circSHKBP1作为癌基因促进癌症进展, 因此我们假设circSHKBP1也参与结肠癌的发展.
探讨circSHKBP1对结肠癌细胞增殖及凋亡的影响及其可能作用机制.
选取本院2020-03/2020-07的69例结肠癌组织及癌旁组织标本, qRT-PCR检测circSHKBP1、miR-125a-5p的表达量; 体外培养人结肠癌细胞HT29, 随机分组: sh-NC组、sh-circSHKBP1组、miR-NC组、miR-125a-5p组、sh-circSHKBP1+anti-miR-NC组、sh-circSHKBP1+anti-miR-125a-5p组; 采用MTT法、平板克隆形成、和流式细胞术检测细胞增殖、克隆形成及凋亡; 采用双荧光素酶报告验证miR-125a-5p过表达对野生型(wild-type, WT)载体wt-circSHKBP1的荧光素酶活性; 采用Western blot检测b细胞淋巴瘤-2(B-cell lymphoma-2, Bcl-2), Bcl-2相关X蛋白(BCL-2-associated X protein, Bax)蛋白表达.
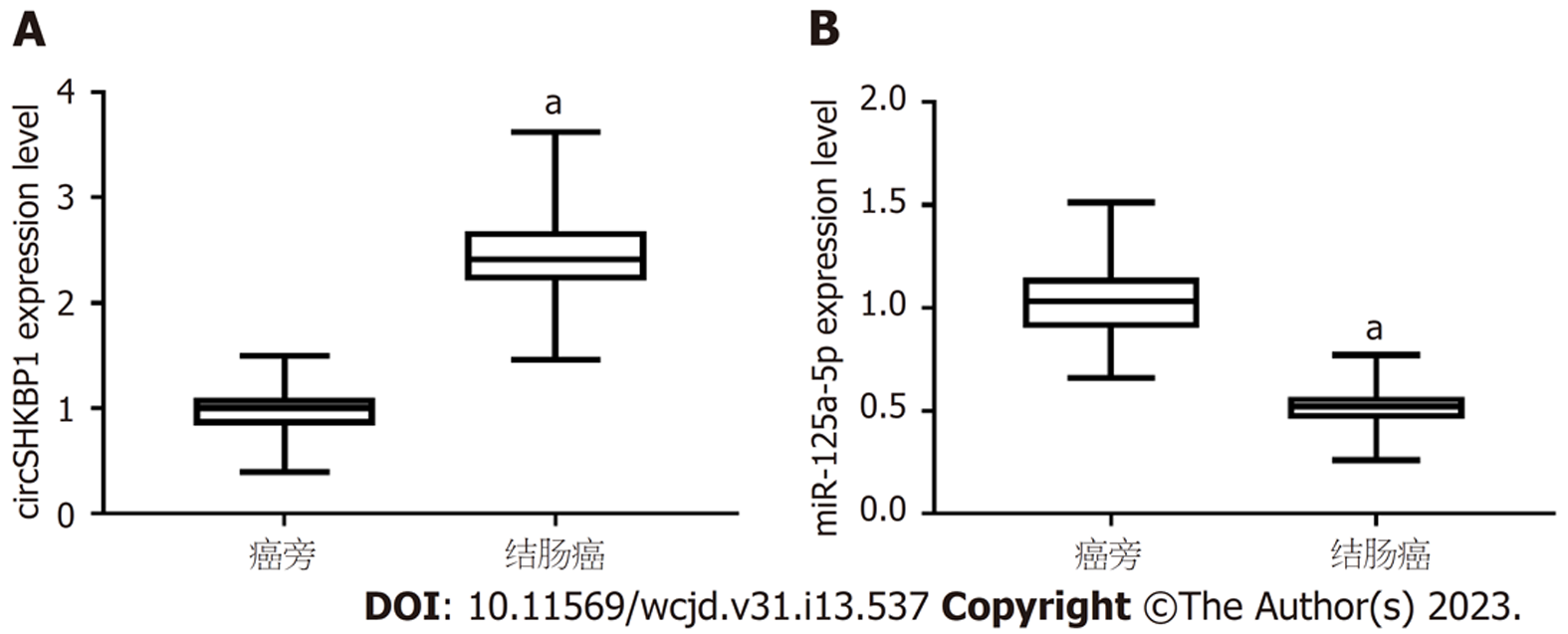
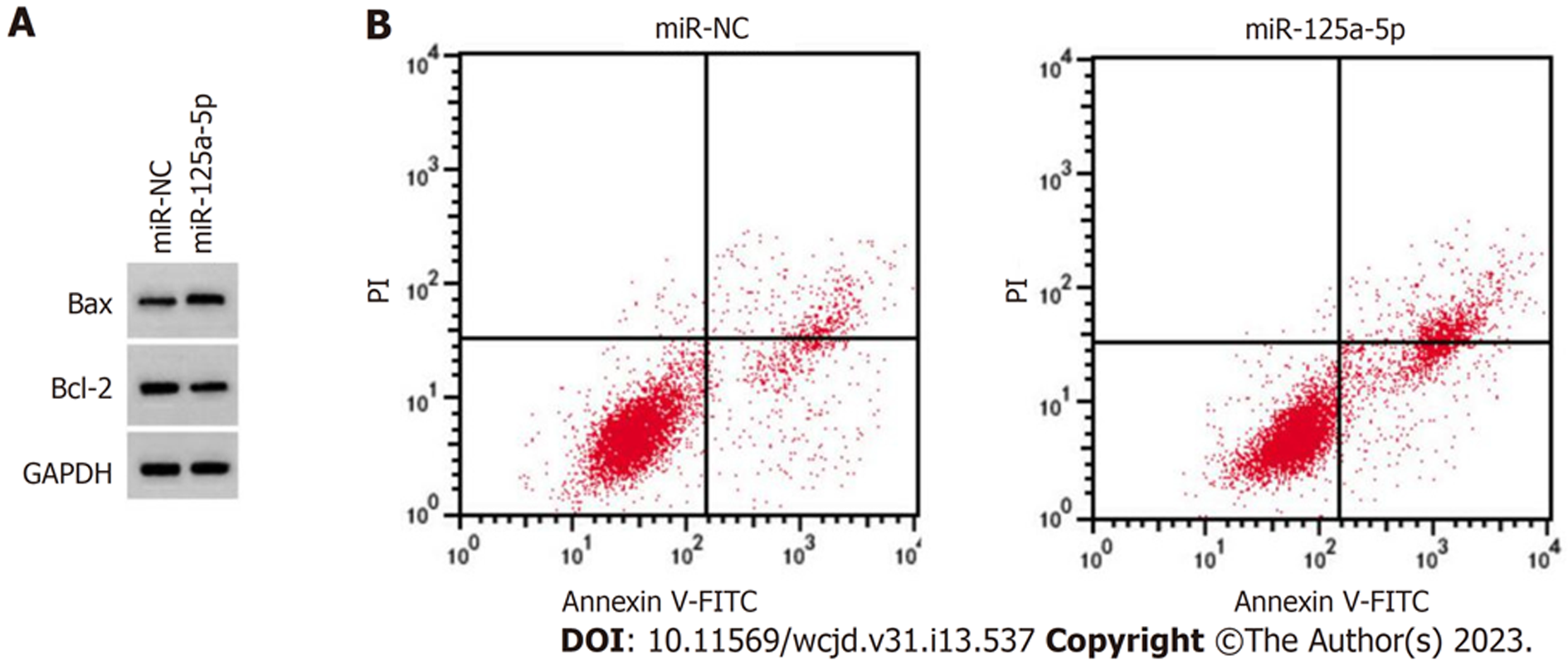
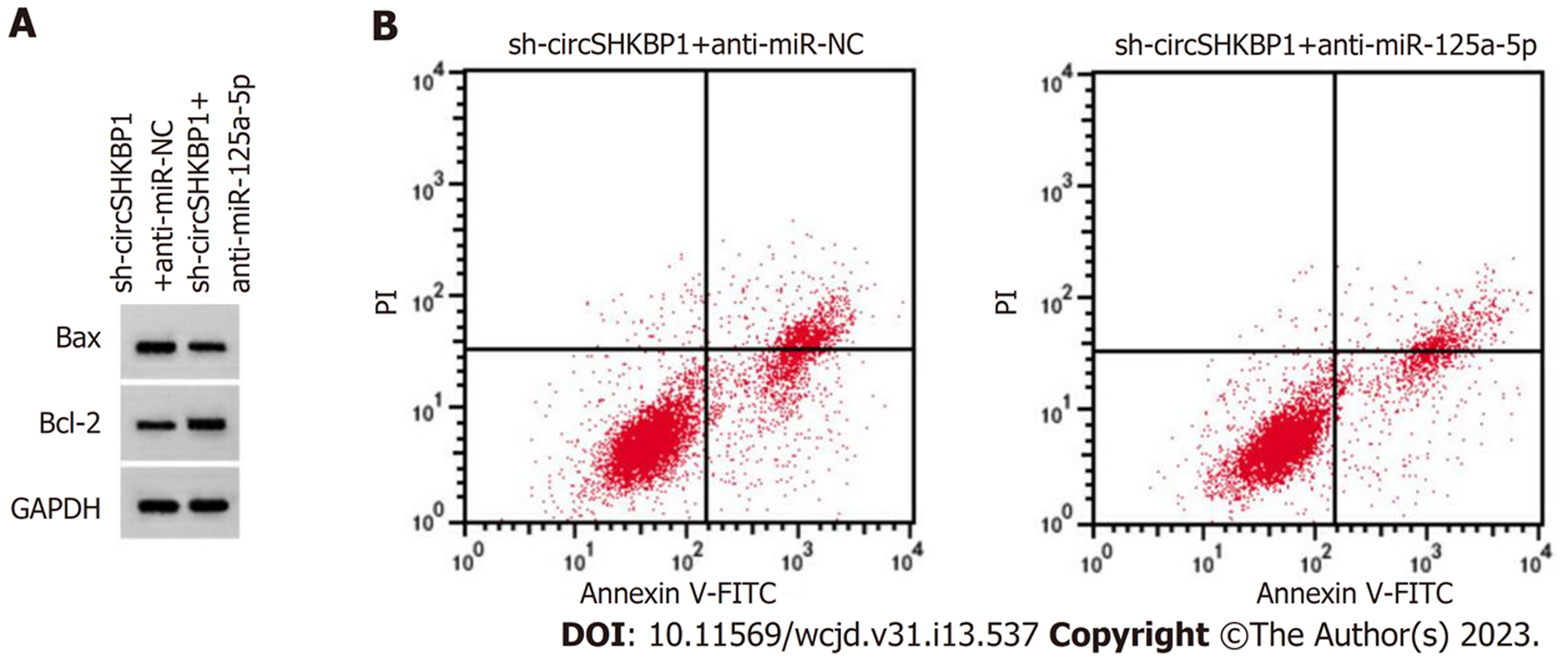
在结肠癌组织中circSHKBP1的表达上调(P<0.05), miR-125a-5p的表达下降(P<0.05); 相对sh-NC组, 沉默circSHKBP1显著降低结肠癌细胞的增殖率、提高凋亡率和Bax的表达, 降低了细胞克隆形成数, 减少Bcl-2的表达(P<0.05); miR-125a-5p过表达可降低wt-circSHKBP1的荧光素酶活性(P<0.05); 与miR-NC组对照, miR-125a-5p组降低增殖率、提高凋亡率和Bax的表达, 减少细胞克隆形成数, 降低Bcl-2的表达(P<0.05); 相对sh-circSHKBP1+anti-miR-NC组, sh-circSHKBP1+anti-miR-125a-5p组提高了肿瘤细胞增殖率、降低了凋亡率和Bax的表达, 增加了细胞克隆形成数, 提高了Bcl-2的表达(P<0.05).
敲低circSHKBP1可通过靶向miR-125a-5p表达抑制结肠癌细胞增殖并促进细胞凋亡.
核心提要: 有研究报道, circSHKBP1可作为促癌基因参与癌症进展, 因此我们假设circSHKBP1也参与结肠癌的发展. 随后我们证实敲低circSHKBP1可通过靶向miR-125a-5p表达抑制结肠癌细胞增殖并促进细胞凋亡.
引文著录: 杨廷旭, 薛蕊芳. circSHKBP1通过靶向miR-125a-5p调控结肠癌细胞增殖和凋亡. 世界华人消化杂志 2023; 31(13): 537-543
Revised: June 10, 2023
Accepted: June 29, 2023
Published online: July 8, 2023
Colon cancer is a common malignant tumor in clinical practice. It has been reported that circular RNAs (circRNAs) are involved in the occurrence and development of cancer. Among them, circSHKBP1 acts as an oncogene to promote cancer progression. Thus, we hypothesized that circSHKBP1 might be also implicated in the development of colon cancer.
To explore the role of circSHKBP1 in colon cancer cell proliferation and apoptosis and the possible mechanism involved.
Sixty-nine cancer tissues and matched adjacent normal tissues were selected from March 2020 to July 2020 at our hospital. The expression of circSHKBP1 and miR-125a-5p was detected by qRT-PCR. Human colon cancer cells (HT29) cultured in vitro were randomly divided into sh-NC group, sh-circSHKBP1 group, miR-NC group, miR-125a-5p group, sh-circSHKBP1 + anti-miR-NC group, and sh-circSHKBP1 + anti-miR-125a-5p group. MTT assay, colony formation experiment, and flow cytometry were used to detect cell proliferation, colony formation, and apoptosis, respectively. The dual luciferase reporter experiment was used to detect the impact of miR-125a-5p overexpression on the luciferase activity of the wild-type vector wt-circSHKBP1. Western blot was used to detect the protein expression of Bax and Bcl-2.
Compared with adjacent tissues, the expression of circSHKBP1 in colon cancer tissues was increased (P < 0.05), while the expression of miR-125a-5p was decreased (P < 0.05). Cell proliferation inhibition rate, apoptosis rate, and Bax protein level in the sh-circSHKBP1 group were increased (P < 0.05), while the number of cell colonies (P < 0.05) and Bcl-2 protein level were decreased (P < 0.05). Overexpression of miR-125a-5p could reduce the luciferase activity of wt-circSHKBP1 (P < 0.05). Relative to the miR-NC group, miR-125a-5p reduced cell proliferation, increased apoptosis rate and Bax protein level (P < 0.05), decreased the number of cell colonies (P < 0.05), and reduced Bcl-2 protein level (P < 0.05). Compared with the sh-circSHKBP1 + anti-miR-NC group, cell proliferation inhibition rate, apoptosis, and the protein level of Bax in the sh-circSHKBP1 + anti-miR-125a-5p group were decreased (P < 0.05), while the number of cell colonies and Bcl-2 protein level were increased (P < 0.05).
Knockdown of circSHKBP1 could inhibit colon cancer cell proliferation and promote apoptosis via up-regulating miR-125a-5p expression.
- Citation: Yang TX, Xue RF. CircSHKBP1 regulates colon cancer cell proliferation and apoptosis by targeting miR-125a-5p. Shijie Huaren Xiaohua Zazhi 2023; 31(13): 537-543
- URL: https://www.wjgnet.com/1009-3079/full/v31/i13/537.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v31.i13.537
结肠癌是临床上常见的一种恶性肿瘤, 我国结肠癌发病率与死亡率逐年上升, 已严重威胁患者生命安全, 随着医疗技术的进步, 分子靶向治疗等成为结肠癌的主要治疗手段, 因而寻找结肠癌发生发展相关基因对提高结肠癌的治疗效果具有重要意义[1,2]. 环状RNA(circular RNA, circRNA)是不具有5'端帽子结构与3'端多聚腺苷酸尾巴结构的非编码RNA分子, 其具有稳定性、特异性等特点, 且它可靶向微小RNA(microRNA, miRNA/miR)调控miRNA靶基因的表达, 进而发挥生物学功能, 与结肠癌进展密切相关[3,4]. 有报道称, circSHKBP1在胃癌细胞中表达上调, 并可通过调控miR-582-3p/HUR/VEGF表达而促进胃癌细胞转移[5]. 然而, 目前尚不明确circSHKBP1在结肠癌的作用. 通过应用 Starbase分析circSHKBP1同miR-125a-5p能够通过结合位点结合. 相关的文献证实了miR-125a-5p可减少结肠癌细胞增殖和诱导凋亡[6]. 但尚不清楚circSHKBP1/miR-125a-5p分子轴对结肠癌的影响. 本研究着重验证是否circSHKBP1可以调节结肠癌细胞增殖及凋亡通过靶向miR-125a-5p.
收集本院2020-03/2020-07经病理学确诊的69例结肠癌患者的肿瘤及癌旁组织, 术后标本立即置于-80 ℃保存. 男39例, 女30例, 年龄(48-67)岁, 平均年龄(53.21±4.16)岁. 本研究符合《世界医学协会赫尔辛基宣言》相关要求, 所有受试者均签署知情同意书. 本研究已征得本院伦理委员会批准.
人结肠癌HT29细胞购自上海晶抗生物; 于美国Gibco购置DMEM培养液与胎牛血清; 于美国Invitrogen 购买Trizol试剂(cat. no. 15596-018)、Lipofectamine2000转染试剂(cat. no. 11668-019); 北京天根生化提供反转录(cat. no. KR107)与荧光定量PCR试剂盒(cat. no. KR123); 于广州锐博生物购买了sh-NC、sh-circSHKBP1、miR-NC、miR-125a-5p mimics、anti-miR-NC、anti-miR-125a-5p; 北京索莱宝提供了MTT试剂盒(cat. no. M1020)、细胞凋亡检测试剂盒(cat. no. CA1040)与荧光素酶活性检测试剂盒; 于美国Promega购置双荧光素酶报告基因载体; 于美国CST和Abcam购置了兔抗人Bax、Bcl-2、GAPDH抗体及HRP标记的山羊抗兔IgG二抗.
1.2.1 实验分组: 接种于6孔板(1×105个/孔)中, 待HT29细胞生长至80%融合度时进行转染. 依据脂质体转染法, 将sh-NC、sh-circSHKBP1、miR-NC、miR-125a-5p mimics分别对细胞进行转染, 记为sh-NC组、sh-circSHKBP1组、miR-NC组、miR-125a-5p四组. 此外, 将sh-circSHKBP1分别与anti-miR-NC和anti-miR-125a-5p对细胞共转, 标记成sh-circSHKBP1+anti-miR-NC组和sh-circSHKBP1+anti-miR-125a-5p组.
1.2.2 qRT-PCR检测circSHKBP1、miR-125a-5p的表达水平: 依据Trizol试剂, 总RNA来自组织和细胞被提取并测定其浓度使用紫外分光光度计. 通过反转录合成cDNA, 随后进行qRT-PCR扩增, 引物序列: circSHKBP1上游5'-CTTGTCAGCGAGCTCTATCG-3', 下游5'-GTAAATGGAGCCGTTGTTGC-3'; miR-125a-5p上游5'-TCGGCAGGTCCCTGAGACCCTT-3', 下游5'-CTCAACTGGTGTCGTGGA-3'; U6上游5'-TCCGACGCCGCCATCTCTA-3', 下游5'-TATCGCACATTAAGCCTCTA-3'; β-actin上游5'-GGCCCAGAATGCAGTTCGCCTT-3', 下游5'-AATGGCACCCTGCTCACGCA-3'. 反应程序: 95 ℃预变性2 min, 95 ℃变性30 s, 60 ℃退火30 s, 72 ℃延伸30 s, 共40个循环. 通过罗氏LightCycler480荧光定量PCR仪, circSHKBP1、miR-125a-5p相对表达量被评估.
1.2.3 MTT检测细胞增殖: 取对数生长期HT29细胞接种于96孔板(2×103个/孔)后, 将20 μL MTT试剂添加到每孔继续常温培养4 h, 弃上清, 将150 μL DMSO加入避光振荡. 5 min后, 酶标仪490 nm处检测吸光度值(A)并计算细胞增殖抑制率[(对照组OD-实验组OD)/(对照组OD-空白组OD)×100%].
1.2.4 平板克隆形成实验: 取500个HT29细胞于6孔板中, 然后常温培育至出现肉眼可见的细胞克隆团. 预冷PBS洗涤后, 细胞被500 μL甲醇固定20 min, 然后400 μL 1%结晶紫染色15 min. 最后, 细胞克隆形成数被拍照和观察.
1.2.5 细胞凋亡率检测: 胰蛋白酶消化后, HT29细胞被收集, 用预PBS洗涤. 随后, 添加 500 μL结合缓冲液对细胞进行重悬, 然后再加入5 μL Annexin V-FITC室温5 min. 检测前加入5 μL PI混匀并放置5 min, 通过FACS Calibur流式细胞仪验证细胞凋亡的情况.
1.2.6 双荧光素酶报告实验检测circSHKBP1与miR-125a-5p的靶向关系: 首先荧光素酶报告基因载体包括: 野生型载体wt-circSHKBP1和缺失miR-125a-5p结合区域的突变型载体mut-circSHKBP1被构建. 依据脂质体转染法, miR-NC或miR-125a-5p mimics分别与wt/mut-circSHKBP1共转染入HT29细胞. 培养24 h, 取上清, 检测细胞荧光素酶活性. 基于脂质体转染法, sh-NC、sh-circSHKBP1分别转染至HT29细胞, 培养48 h, miR-125a-5p的表达量被qRT-PCR分析.
1.2.7 Western blot检测Bax、Bcl-2蛋白表达量: 依据RIPA裂解液, 各组被提取细胞总蛋白, 随后BCA法对蛋白浓度进行统一定量. 5×SDS上样缓冲液被添加, 沸水中煮10 min. 每孔40 μg蛋白样品进行SDS-PAGE, 转膜后室温封闭, 加入一抗Bax(1:1000)、Bcl-2(1:1000)与GAPDH抗体(1:2000), 4 ℃孵育过夜. 将膜浸泡在二抗稀释液(1:3000), 1 h后, 曝光显影, 并分析条带灰度值依据ImageJ软件.
统计学处理 符合正态分布的数据被SPSS 21.0统计学软件分析, 并以(mean±SD)表示. 独立样本t检验作两组间比较, 单因素方差分析作多组间比较, P<0.05表明具有统计学意义.
与癌旁组织比较, 在结肠癌组织中circSHKBP1的表达量提高(P<0.05), miR-125a-5p的表达量降低(P<0.05), 见图1.
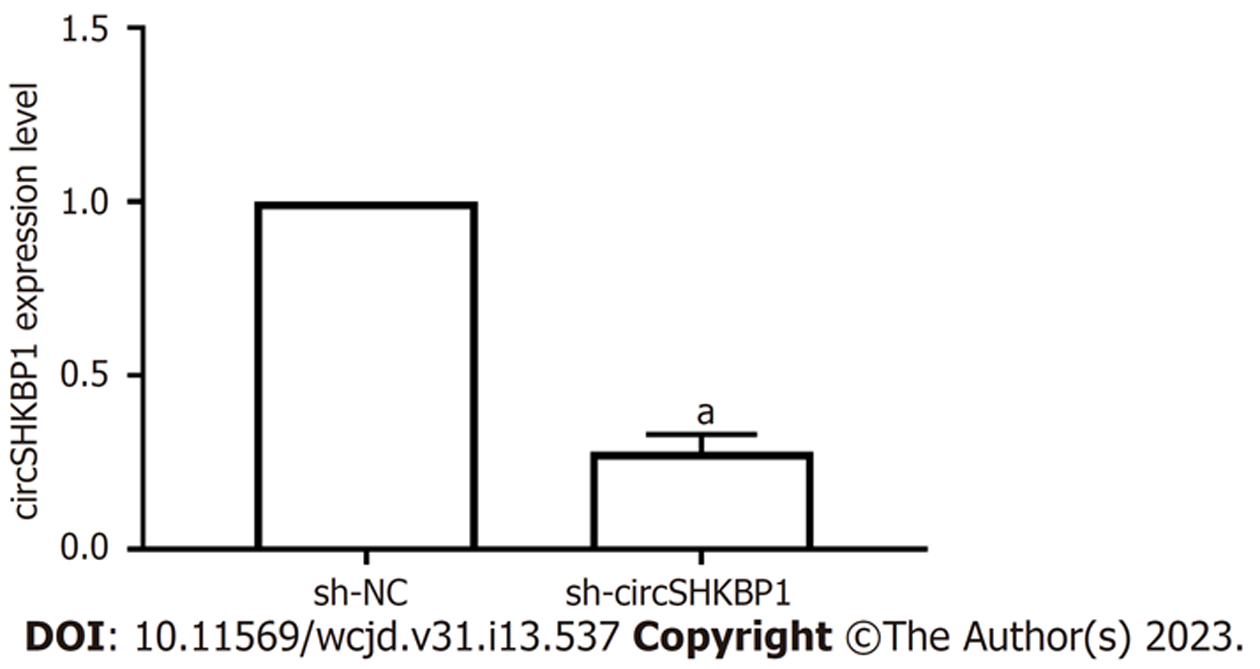
与sh-NC组比较, sh-circSHKBP1组中circSHKBP1的表达量降低(P<0.05), 见图2. 表明转染效果良好并可用于后续实验.
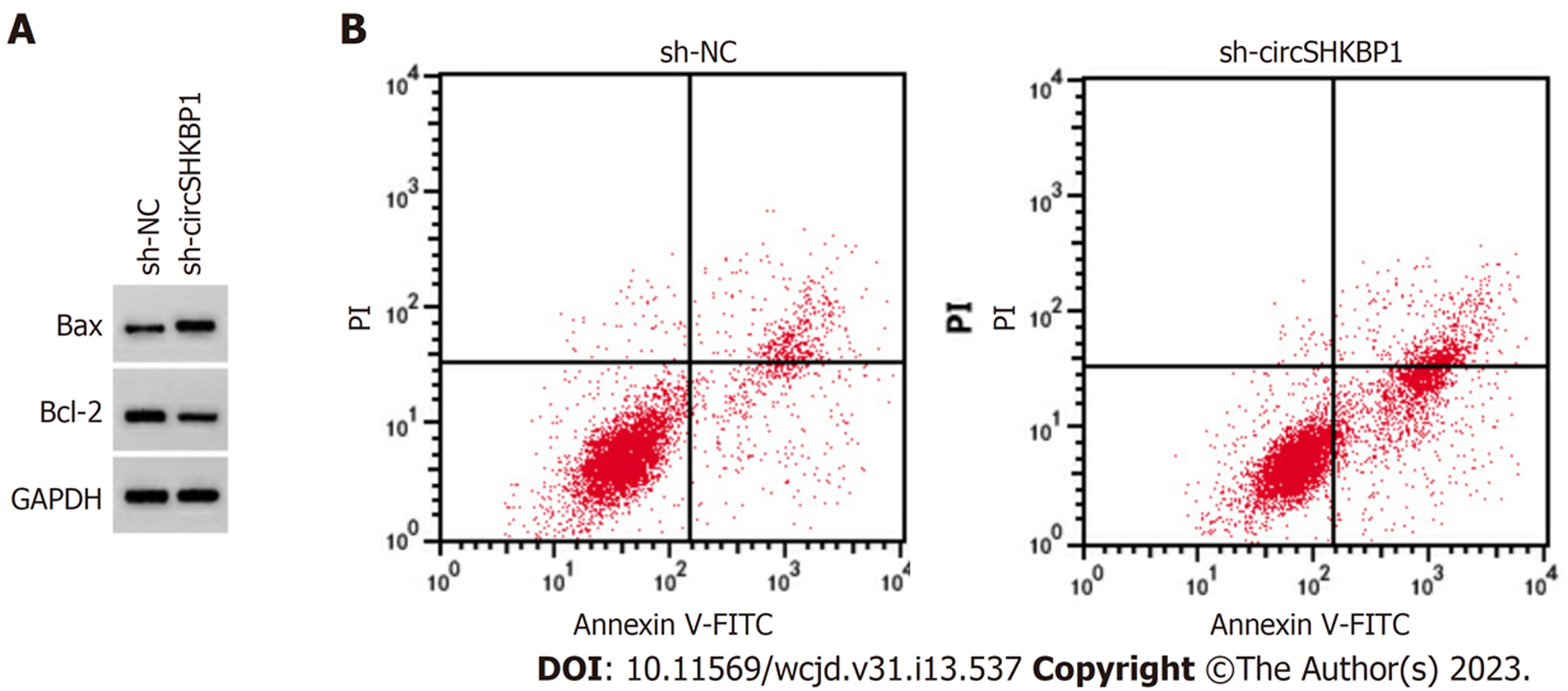
与sh-NC组比较, 沉默circSHKBP1可以提高细胞增殖抑制率、凋亡率和Bax表达水平(P<0.05), 减少克隆形成数(P<0.05), 降低Bcl-2表达水平(P<0.05), 见图3、表1.
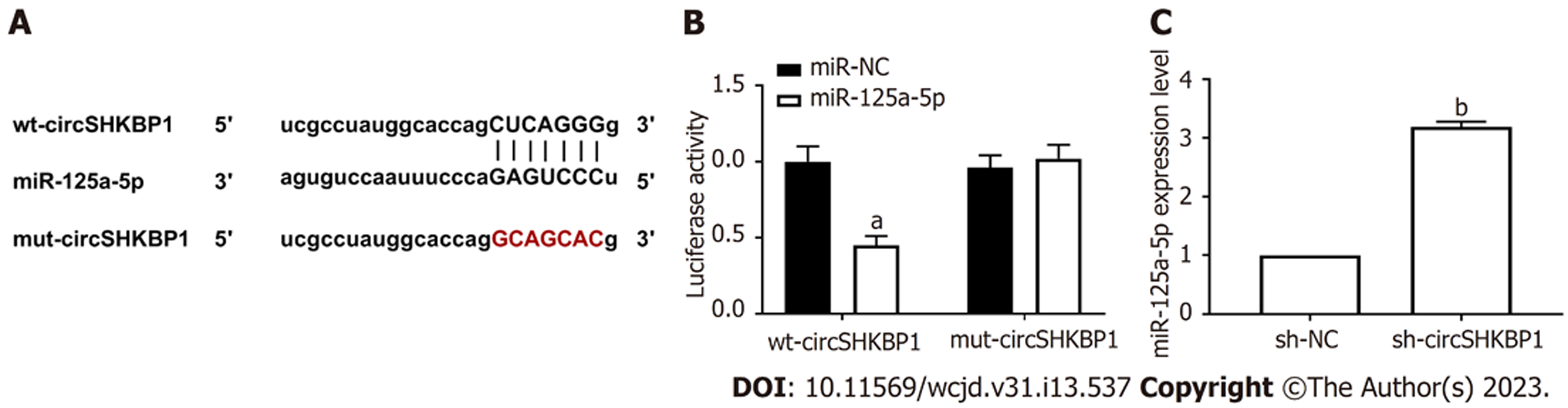
正如图4A所展示, circSHKBP1和miR-125a-5p存在结合位点. 我们的结果显示miR-125a-5p过表达减少野生型载体wt-circSHKBP1荧光素酶活性(P<0.05), 而对变型载体mut-circSHKBP1的荧光素酶活性无明显影响, 见图4B. 与sh-NC组比较, sh-circSHKBP1组中miR-125a-5p水平明显升高(P<0.05), 见图4C.
与miR-NC组对比, miR-125a-5p组可以提高细胞增殖抑制率、凋亡率和Bax表达水平(P<0.05), 抑制克隆形成数(P<0.05), 减少Bcl-2表达水平(P<0.05), 见图5、表2.
与sh-circSHKBP1+anti-miR-NC组对比, sh-circSHKBP1+anti-miR-125a-5p组可以降低细胞增殖抑制率、凋亡率和Bax(P<0.05), 增加克隆形成数(P<0.05), 提升Bcl-2(P<0.05), 见图6、表3.
circRNA可通过miRNA表达而正向调控靶基因的表达从而调节结肠癌细胞生物学行为, 其表达异常与结肠癌发生及发展密切相关, 并可能作为结肠癌靶向治疗的相关靶点[7,8]. 在结肠癌组织或细胞系中circRNA表达可以上调或下调, circRNA表达上调时可作为癌基因而促进结肠癌的发展, 而circRNA表达下调时可作为抑癌基因而抑制结肠癌的发展进程[9,10].
circSHKBP1在胶质瘤细胞中表达水平升高, 并可通过调控miR-544a/FOXP1表达而促进胶质瘤细胞血管生成[11]. 然而, 尚不清楚其在结肠癌中的表达及对细胞生物学行为的影响. 本研究结果显示, circSHKBP1是显著增加的在结肠癌组织中, 其沉默可以提高结肠癌细胞增殖抑制率, 减少克隆形成数, 暗示结肠癌细胞增殖及克隆形成可被干扰circSHKBP1抑制. 信服的证据提示了失衡的细胞增殖与凋亡可加剧结肠癌进展, 其中凋亡相关蛋白Bcl-2/Bax比值降低增加凋亡[12,13]. 本研究结果显示, 沉默circSHKBP1表达可增强结肠癌细胞凋亡能力, 并可促进Bax表达而抑制Bcl-2表达, 提示干扰circSHKBP1表达可促进结肠癌细胞凋亡.
本研究结果显示, circSHKBP1被证实可以靶向负调控miR-125a-5p. miR-125a-5p过表达可抑制结肠癌细胞增殖、迁移及侵袭[14]. miR-125a-5p表达上调可通过调控TAZ/EGFR信号通路而抑制卵巢癌细胞转移[15]. 相关的研究证明miR-125a-5p对结直肠癌细胞迁移及侵袭的抑制作用[16]. 本研究验证, miR-125a-5p可加剧结肠癌细胞凋亡和减少增殖及克隆形成, 而其抑制可缓解沉默circSHKBP1引发的增殖、克隆形成抑制和凋亡促进. 表明circSHKBP1可促进结肠癌发展通过靶向miR-125a-5p.
综上所述, 结肠癌组织中circSHKBP1表达上调, miR-125a-5p表达下调, 干扰circSHKBP1可通过靶向下调miR-125a-5p表达调控结肠癌细胞的增殖和凋亡. 这些发现意味着circSHKBP1/miR-125a-5p轴很有可能为结直肠癌的治疗提供新的分子靶点, 但这些研究只仅限于体外的实验. 关于circSHKBP1在体内是如何作用于结直肠癌以及相关的作用机制仍有待继续研究.
CircSHKBP1可作为促癌基因参与癌症进展, 因此我们假设circSHKBP1也参与结肠癌的发展.
本文拟研究circSHKBP1在结肠癌细胞生长中的作用, 通过揭示一种新的调控途径, 为结肠癌的治疗提供了新的见解及治疗靶标.
结肠癌是临床上常见的一种恶性肿瘤且在我国发病率与死亡率逐年上升. 我们的目的是研究一个关键的circSHKBP1-miRNA通路在结肠癌中的作用.
采用MTT法、平板克隆形成、和流式细胞术检测细胞增殖及凋亡; 采用双荧光素酶报告实验探索circSHKBP1-miRNA通路.
结肠癌组织中circSHKBP1高表达上调, miR-125a-5p低表达, 敲除实验证实敲低circSHKBP1可通过靶向调控miR-125a-5p表达抑制结肠癌细胞增殖, 促进细胞凋亡, 结果表明, 致癌circSHKBP1通过竞争性结合miR-125a-5p来驱动癌症进展.
本次研究首次证实circSHKBP1在结肠癌中作为致癌基因, 通过靶向抑制miR-125a-5p的表达, 促进癌细胞的生长. 我们首次在结肠癌细胞中建立了circSHKBP1-miR-125a-5p通路, 我们的研究结果表明circSHKBP1可能成为结肠癌的潜在治疗靶点, circSHKBP1特异性小干扰RNA可能是用于结肠癌临床治疗的有希望的分子.
虽然在这项工作中发现了一些有趣的结果, 但所提供的数据是基于有限数量的体外细胞. 考虑到本研究的不足, 未来需要进行体内实验和收集更大数量的临床标本来验证这些结论.
学科分类: 胃肠病学和肝病学
手稿来源地: 甘肃省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C, C
D级 (一般): 0
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Zhou P, Xie W, Huang HL, Huang RQ, Tian C, Zhu HB, Dai YH, Li ZY. circRNA_100859 functions as an oncogene in colon cancer by sponging the miR-217-HIF-1α pathway. Aging (Albany NY). 2020;12:13338-13353. [PubMed] [DOI] |
| 2. | Zhang Q, Zhang C, Ma JX, Ren H, Sun Y, Xu JZ. Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a. World J Gastroenterol. 2019;25:5300-5309. [PubMed] [DOI] |
| 3. | Zhao G, Dai GJ. Hsa_circRNA_000166 Promotes Cell Proliferation, Migration and Invasion by Regulating miR-330-5p/ELK1 in Colon Cancer. Onco Targets Ther. 2020;13:5529-5539. [PubMed] [DOI] |
| 4. | Chen Y, Yang F, Fang E, Xiao W, Mei H, Li H, Li D, Song H, Wang J, Hong M, Wang X, Huang K, Zheng L, Tong Q. Circular RNA circAGO2 drives cancer progression through facilitating HuR-repressed functions of AGO2-miRNA complexes. Cell Death Differ. 2019;26:1346-1364. [PubMed] [DOI] |
| 5. | Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y, Xu T. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19:112. [PubMed] [DOI] |
| 6. | Tong Z, Liu N, Lin L, Guo X, Yang D, Zhang Q. miR-125a-5p inhibits cell proliferation and induces apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1. Biomed Pharmacother. 2015;75:129-136. [PubMed] [DOI] |
| 7. | Min L, Wang H, Zeng Y. CircRNA_104916 regulates migration, apoptosis and epithelial-mesenchymal transition in colon cancer cells. Front Biosci (Landmark Ed). 2019;24:819-832. [PubMed] [DOI] |
| 8. | Gao C, Zhang Y, Tian Y, Han C, Wang L, Ding B, Tian H, Zhou C, Ju Y, Peng A, Yu Q. Circ_0055625 knockdown inhibits tumorigenesis and improves radiosensitivity by regulating miR-338-3p/MSI1 axis in colon cancer. World J Surg Oncol. 2021;19:131. [PubMed] [DOI] |
| 9. | Yang S, Gao S, Liu T, Liu J, Zheng X, Li Z. Circular RNA SMARCA5 functions as an anti-tumor candidate in colon cancer by sponging microRNA-552. Cell Cycle. 2021;20:689-701. [PubMed] [DOI] |
| 10. | Pan Z, Cai J, Lin J, Zhou H, Peng J, Liang J, Xia L, Yin Q, Zou B, Zheng J, Qiao L, Zhang L. A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating Snail in colon cancer. Mol Cancer. 2020;19:71. [PubMed] [DOI] |
| 11. | He Q, Zhao L, Liu Y, Liu X, Zheng J, Yu H, Cai H, Ma J, Liu L, Wang P, Li Z, Xue Y. circ-SHKBP1 Regulates the Angiogenesis of U87 Glioma-Exposed Endothelial Cells through miR-544a/FOXP1 and miR-379/FOXP2 Pathways. Mol Ther Nucleic Acids. 2018;10:331-348. [PubMed] [DOI] |
| 12. | Cheng B, Rong A, Zhou Q, Li W. LncRNA LINC00662 promotes colon cancer tumor growth and metastasis by competitively binding with miR-340-5p to regulate CLDN8/IL22 co-expression and activating ERK signaling pathway. J Exp Clin Cancer Res. 2020;39:5. [PubMed] [DOI] |
| 13. | Pang L, Zhang Q, Wu Y, Yang Q, Zhang J, Liu Y, Li R. Long non-coding RNA CCAT1 promotes non-small cell lung cancer progression by regulating the miR-216a-5p/RAP2B axis. Exp Biol Med (Maywood). 2021;246:142-152. [PubMed] [DOI] |
| 14. | Ren Y, Zhao C, He Y, Min X, Xu H, Hu X. RPARP-AS1/miR125a-5p Axis Promotes Cell Proliferation, Migration and Invasion in Colon Cancer. Onco Targets Ther. 2021;14:5035-5043. [PubMed] [DOI] |
| 15. | Cao Y, Shen T, Zhang C, Zhang QH, Zhang ZQ. MiR-125a-5p inhibits EMT of ovarian cancer cells by regulating TAZ/EGFR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:8249-8256. [PubMed] [DOI] |
| 16. | Tang L, Zhou L, Wu S, Shi X, Jiang G, Niu S, Ding D. miR-125a-5p inhibits colorectal cancer cell epithelial-mesenchymal transition, invasion and migration by targeting TAZ. Onco Targets Ther. 2019;12:3481-3489. [PubMed] [DOI] |