修回日期: 2020-09-24
接受日期: 2020-10-26
在线出版日期: 2020-11-28
胰腺癌是人类最具致命的恶性肿瘤之一, 其中胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)是最常见的组织学类型. 由于PDAC早期缺乏特异的临床症状、体征及有效的筛查标志物, 仅15%-20%的患者具备手术切除条件. 因此, 以吉西他滨(gemcitabine, GEM)为基础的单药或联合化疗仍是目前最主要甚或唯一的治疗方案. 然而, PDAC对GEM的总反应率不足20%, 对GEM耐药是影响化疗疗效的最重要原因之一. 目前GEM耐药的机制并不明确, 可能涉及先天性和获得性两个方面, PDAC的异质性则进一步增加了其复杂性. 然而, 细胞内信号通路调控是实现GEM抵抗的最终途径. 本文将重点对GEM在PDAC细胞内的代谢和信号通路调控的研究进展进行综述, 讨论潜在的GEM化疗增敏策略, 以期提高化疗有效率, 改善PDAC的总体预后.
核心提要: 吉西他滨(gemcitabine, GEM)耐药是影响胰腺癌化疗疗效的重要原因. GEM耐药的机制复杂且不明确, 近年来细胞内调控机制研究取得众多新进展, 这将为潜在的GEM化疗增敏策略提供了新的靶点, 对进一步提高化疗有效率, 改善胰腺导管腺癌的总体预后具有重要意义.
引文著录: 顾宗廷, 李宗泽, 王成锋. 胰腺癌细胞内吉西他滨耐药机制的研究进展. 世界华人消化杂志 2020; 28(22): 1150-1161
Revised: September 24, 2020
Accepted: October 26, 2020
Published online: November 28, 2020
Pancreatic cancer is one of the most deadly malignant tumors that endanger human health, and pancreatic ductal adenocarcinoma (PDAC) is the most common histological type. Due to the lack of specific clinical symptoms, physical signs, and effective screening biomarkers for early stage PDAC, only 15%-20% of patients are qualified for surgical resection. Consequently, gemcitabine (GEM)-based monotherapy or combination therapy is still the most important or even the only treatment option. However, the overall response rate of PDAC to GEM is less than 20%, and GEM resistance is one of the most important factors affecting the efficacy of chemotherapy. At present, the mechanism of GEM resistance has not been clarified, which may involve congenital and acquired regulation. The heterogeneity of PDAC further increases its complexity. However, regulation of intracellular signaling pathways is the ultimate event to induce GEM resistance. This article will review the recent advances in research of GEM metabolism and regulation of signaling pathways in PDAC cells, and discuss potential GEM chemosensitization strategies, in order to improve the effective rate of chemotherapy and the outcome.
- Citation: Gu ZT, Li ZZ, Wang CF. Research advances of intracellular mechanisms underlying gemcitabine resistance in pancreatic cancer. Shijie Huaren Xiaohua Zazhi 2020; 28(22): 1150-1161
- URL: https://www.wjgnet.com/1009-3079/full/v28/i22/1150.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v28.i22.1150
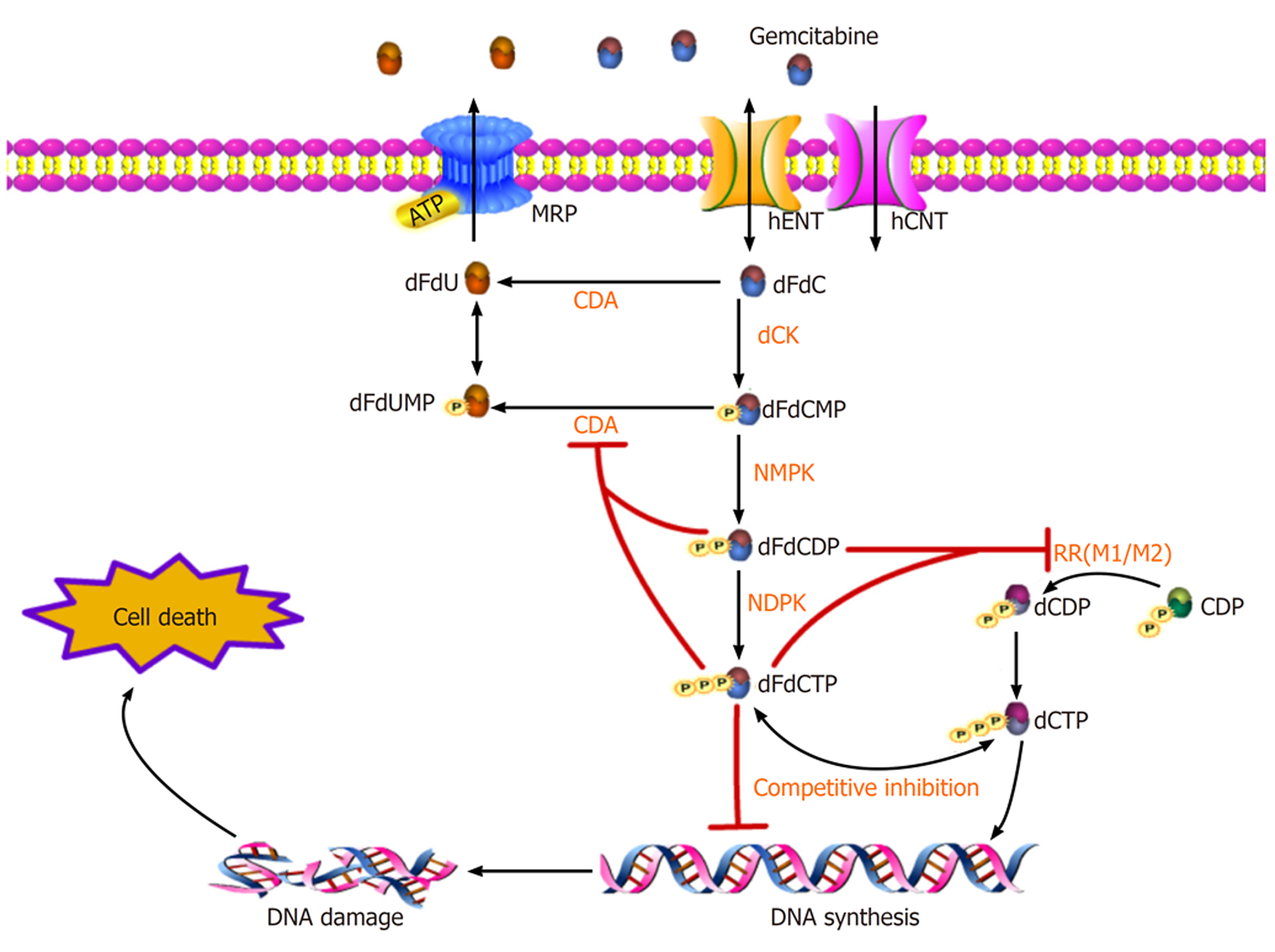
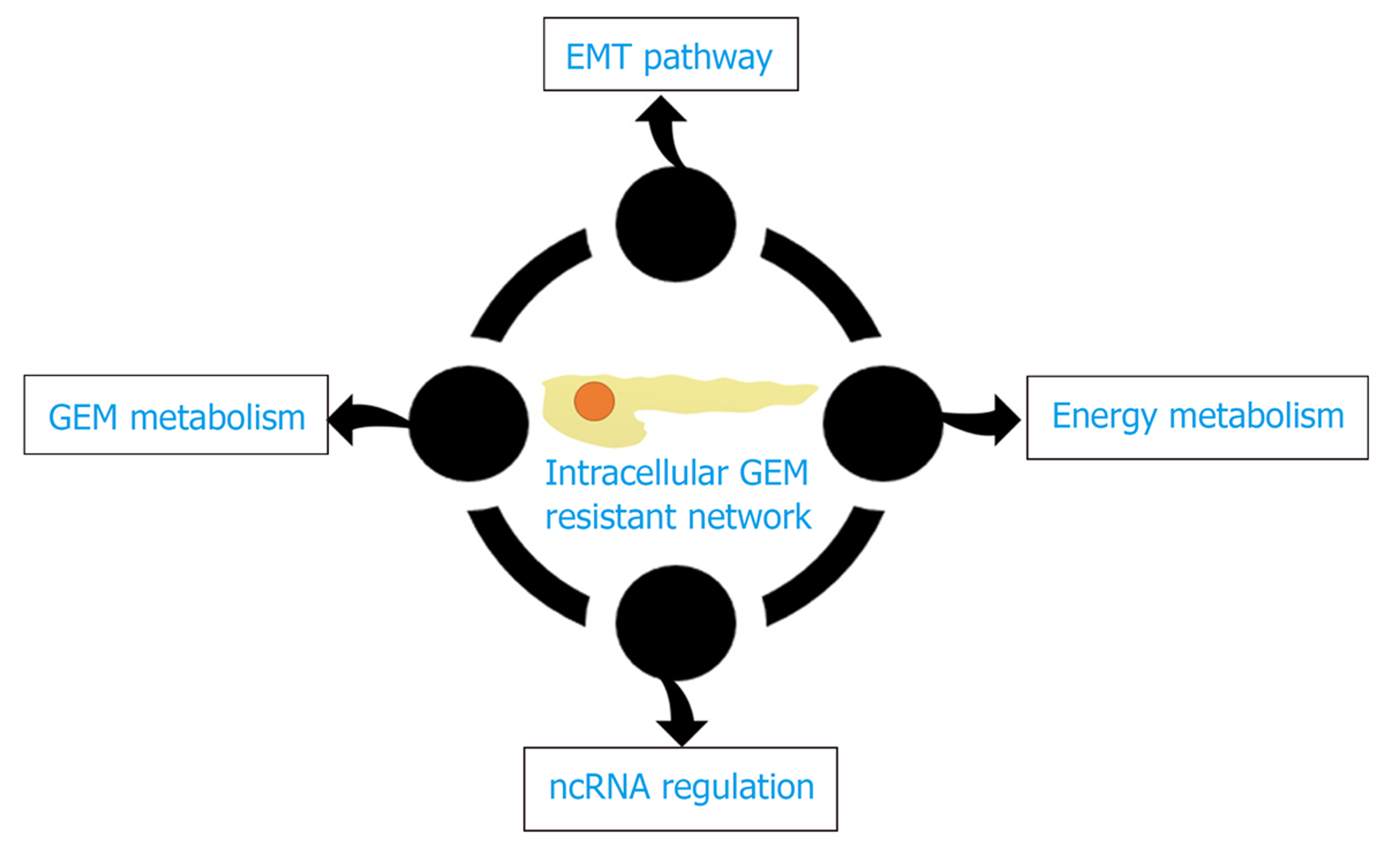
胰腺癌是人类最具致命的恶性肿瘤之一, 胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)是其中最常见的类型, 约占90%. 尽管PDAC治疗技术不断进步, 但在过去40年中生存率改善有限, 其5年生存率仍低于9%, 且在所有实体瘤中预后最差[1]. 目前, 手术切除仍是PDAC患者唯一可能治愈的手段, 但由于缺乏明确的临床症状或体征使得早期诊断变得异常困难. 因此, 仅有15%-20%的患者有手术切除的指征[2], 对于临界可切除、进展期或转移性PDAC而言, 全身化疗(包括新辅助)则是最主要甚或唯一的治疗方案. 其中, 核苷类似物吉西他滨(gemcitabine, GEM)的单药或联合治疗仍是目前PDAC化疗的一线方案[3]. GEM作为一种前药进入PDAC细胞, 经过一系列精密调控的磷酸化过程, 其衍生物可以干扰DNA合成并阻止癌细胞周期的进展[4](图1). 但GEM治疗胰腺癌的总反应率不足20%, 约80%的患者可在1年内因肿瘤转移而死亡[5]. PDAC对GEM的耐药是影响化疗疗效并导致预后不良的最重要原因之一. 因此, 克服GEM耐药仍是PDAC治疗中面临的主要挑战之一[6]. 肿瘤异质性是PDAC耐药性产生的主要原因, 其与肿瘤微环境、遗传物质不稳定、细胞内信号分子调控等密切相关, 因此GEM耐药机制十分复杂, 可能涉及先天性和获得性两个方面, 但细胞内信号通路调控是实现GEM抵抗的最终途径(图2). 本文将重点讨论GEM在PDAC细胞内的代谢和信号通路调控机制的研究进展(表1). 针对这些细胞及分子机制的进一步研究将有助于开发新的化疗增敏策略, 提高GEM化疗有效率, 改善总体预后.
| 潜在靶点 | 功能 | 参考文献 |
| hENT1, hENT2, hCNT1, hCNT3 | 介导dFdC细胞内转运 | [7,9-12,15] |
| MUC4 | 通过NF-κB负性调控hCNT1表达 | [17] |
| MRP1, MRP5 | 介导GEM外排 | [19-21] |
| dCK | dFdC细胞内代谢限速酶 | [25,26] |
| RR | DNA合成限速酶 | [28-30] |
| CDA | 通过脱氨作用灭活dFdC | [33,34] |
| ERCC1 | 修复GEM诱发的DNA链断裂 | [36] |
| TOP-I | 干扰DNA复制或转录 | [8] |
| BCL-xl, MCL-1, BAX, BAK | 凋亡调控基因 | [41-43] |
| BH3-only | 促凋亡蛋白 | [44,45] |
| caspase-9/-3/-7 | 激活凋亡下游级联信号 | [46] |
| P53 | 维持基因组完整性和调控凋亡 | [48,49] |
| VMP1 | 诱导自噬 | [54] |
| Beclin-1 | 激活ROS/Akt/mTOR通路; 诱导凋亡 | [55] |
| Kras | 通过Kras/ERK通路抑制Ferroptosis; 下调dCK, hENT1表达 | [58] |
| ROS | 维持CSC和EMT表型 | [61,62] |
| MUC1, HIF-1α, PPP, Nampt | 正性调控有氧糖酵解 | [63,67] |
| FBP1, USP44, c-Myc, TXNIP | 负性调控有氧糖酵解 | [65,66] |
| TIGAR | 调控TP53诱导的糖酵解和凋亡 | [68] |
| N-glycosylation | 参与TGF-β, TNF, NF-κB促生存通路的活化 | [70] |
| LAT2 | 激活Gln依赖的mTOR通路, 促进糖酵解, 抑制凋亡 | [71] |
| Gln | 促进N-glycosylation和HBP | [72] |
| Met | 细胞增殖的Met依赖作用 | [73] |
| Ser | 核苷酸和氨基酸合成底物 | [74] |
| FASN | 调节脂质合成; 促进糖酵解, 抑制凋亡 | [77-79] |
| ω3-PUFA | 抑制NF-κB, Akt/STAT3促生存通路活化 | [80,81] |
| LDLR | 促进胆固醇摄取 | [82] |
| E-cadherin, ZEB-1 | EMT转化表型 | [88] |
| miRNA-410-3p/-146a-5p | 激活自噬, NF-κB促生存通路; 促进EMT转化 | [97-99] |
| circRNA | miRNA海绵作用 | [100,101] |
GEM是一种脱氧胞苷核苷类似物(2′,2′-difluorodeoxycytidine, dFdC),其靶点位于细胞内. 由于与核苷的结构相似, dFdC的摄取由核苷转运体(nucleoside transporter, NTs)负责, NTs分为浓缩核苷转运体(human concentrative nucleoside transporters, hCNTs)和平衡核苷转运体(human equilibrative nucleoside transporters, hENTs)两大类[7]. dFdC的细胞内转运主要由hENT1介导, 也有少量由hENT2、hCNT1和hCNT3介导[8](图1). 之前的研究表明, hENT1的高表达与胰腺癌患者更长的总生存期(overall survival, OS)和无病生存期(disease-free survival, DFS)有关, hENT1的表达水平可作为GEM化疗的胰腺癌切除患者的预后评价指标[9]. 通过增加hENT1的表达来促进dFdC的细胞内摄取是一种已知的化疗增敏机制[7]. 此外, hENT1活性也是癌细胞对GEM敏感性的重要决定因素, 因此hENT1的缺乏或活性降低可能是导致GEM耐药的原因之一[10]. 另外, 药物抑制hCNT1降解也增加了dFdC的转运, 改善GEM的药效[11]. hCNT3高表达也与OS延长有关[12]. 遗憾的是, 最近有一项随机临床试验(JASPAC 01)显示, GEM组中ENT1的高表达与OS无关[13]. 有趣的是, GEM的饱和吸收是由其高亲和力和低亲和力成分介导的, 高亲和力和低亲和力位点分别对应与hENT1和hENT2结合[14]. 最近一项有关PDAC伴肝转移行动脉灌注GEM化疗的研究显示, GEM的肝脏摄取主要由在不饱和条件下的hENT2决定, 提示hENT2的表达水平可作为PDAC伴肝转移患者选择动脉灌注GEM化疗的标志物[15]. 此外, MUC4作为一种高分子量的跨膜黏蛋白(Mucin, MUC)在PDAC中高表达[16], MUC4蛋白可通过NF-κB途径负调控hCNT1转运蛋白的表达, MUC4的沉默通过增加hCNT1的表达提高对GEM的敏感性[17].
GEM化疗耐药的另一机制是调节药物外排. 多药耐药相关蛋白(multidrug resistance protein, MRP)是一类ATP结合盒(ATP-binding cassette, ABC)转运泵, 其逆浓度梯度跨膜转运的能量来自ATP的水解, 它介导GEM等一系列化疗药的外流, 从而降低细胞内药物浓度并促进耐药发生[18](图1). 在PDAC中, MRP1、MRP5是GEM外排的主要转运体, 且在PDAC细胞系中GEM诱导其表达呈时间和剂量依赖性[19]. MRP1抑制剂研究已成为化疗增敏的重要策略[20,21]. 最新一项研究表明, 阿霉素可作为荧光报告分子, 用于识别新型的MRP1抑制剂[21]. 总之, 对GEM流入/流出的蛋白调控的进一步研究有助于发现新的抗GEM耐药靶点.
图1显示了dFdC的细胞内代谢过程. dFdC进入细胞膜后, 被磷酸化限速酶脱氧胞苷激酶(deoxycytidine kinase, dCK)连续磷酸化成dFdC一/二/三磷酸核苷(dFdCMP/ dFdCDP/ dFdCTP). 其中, dFdCTP 是DNA合成原料三磷酸脱氧胞苷(deoxycytidine triphosphate, dCTP)的竞争性底物, DNA聚合酶在复制中将竞争性dFdCTP掺入DNA中, 终止DNA链的延长, 并最终导致细胞死亡[22]. 有趣的是, DNA聚合酶允许在将dFdCTP与DNA链结合后再配对一个核苷酸, 导致核酸外切酶不能识别和修复DNA, 这一过程又被称之为"终止链屏蔽"[8]. 同时, dFdCDP和dFdCTP这两种活性代谢物能有效抑制核糖核酸还原酶(ribonucleotide reductase, RR), RR是DNA合成途径中的限速酶(RRM1、RRM2), 主要负责将核糖核苷酸转化为对DNA组装和修复至关重要的三磷酸脱氧核糖核苷(deoxyribonucleoside triphosphates, dNTPs), 其抑制作用导致DNA合成所需的原料dCTP池浓度降低, 从而进一步促进dFdCTP竞争性结合DNA. 另外, dFdCDP和dFdCTP还可抑制dCMP脱氨酶(cytidine deaminase, CDA), 后者可将dFdCMP分解为无活性的单磷酸二氟脱氧尿苷(dFdUMP)[23]. 通过上述独特的正反馈的机制, 既减少了竞争性的dCTP池, 又减少了分解代谢, 极大维持了细胞内GEM的活性和有效浓度.
作为dFdC细胞内代谢的关键限速酶, dCK和RR在化疗耐药中的作用已逐渐被揭示. 体外和体内试验均证实dCK活性与GEM敏感性呈正相关[24]. 敲除dCK基因可导致GEM耐药, 而过度表达dCK基因则可恢复PDAC细胞对GEM的化疗敏感性[25]. 临床免疫组化亦证实, 胰腺癌组织中dCK的表达水平是术后GEM化疗患者的预后影响指标之一[26]. 一项最新的研究将脱氧胞苷(deoxycytidine, dC)作为化学交换饱和转移(chemical exchange saturation transfer, CEST)MRI的成像探针用于检测dCK活性, 这为临床评估GEM抗性和预测疗效提供了可能[27]. 与之类似, RR抑制也是GEM敏感性增强的重要机制. PDAC细胞系中 RRM1、RRM2蛋白过度表达可获得稳定的GEM抗性[28]. 最近的一项Meta分析显示, 相对于RRM1高表达的GEM化疗患者, 低表达的患者有更长的OS和DFS[29]. 因此, RR也是预测胰腺癌GEM耐药的潜在标志物[30]. 此外, CDA在GEM化疗耐药中的作用也逐渐引起重视[31,32]. 最近一项研究显示, 在诱导GEM耐药的PDAC细胞系中, CDA的表达显著增加, CDA过表达与GEM的耐药性有关[33], 而CDA下调则与GEM早期毒性反应相关[34]. 因此, CDA过表达可能是GEM化疗耐药性的标志, 且CDA有望成为增强GEM化疗敏感性的新靶标[32].
dFdCTP竞争性结合DNA后, PDAC细胞周期过程被阻断在G1/S相, 机制主要是其诱导的DNA(可能还有RNA)损伤, 且在细胞水平上无法被修复[35]. 虽然"终止链屏蔽"导致核酸外切酶不能识别和修复这种DNA, 但实际上, 仍有一些切除修复酶能够解决这一问题, 其中包括DNA修复内切酶ERCC1(ERCC Excision Repair 1), 其主要负责DNA断裂双链的切割. 研究显示, ERCC1在GEM耐药患者中过表达, 且可修复GEM诱发的链断裂[36]. 除了"终止链屏蔽"直接机制中止DNA链的延长之外, GEM还可通过间接机制导致DNA链断裂. GEM竞争性结合DNA后可以捕获拓扑异构酶I裂解复合体, 并增强其稳定性. 拓扑异构酶I(topoisomerase I, TOP-I)是参与DNA复制、转录等多种遗传过程的关键酶之一. TOP-I裂解复合体的稳定性可干扰DNA复制或转录, 其机制可能是TOP-I裂解复合体与复制或转录复合体之间碰撞频率的增加导致了核酸链断裂不断的累积, 最终造成整个DNA链的断裂[37]. 这种作用又被称为"TOP-I中毒". GEM所致的TOP-I中毒造成了DNA链的断裂并把细胞导向了死亡, 因此TOP-I是GEM耐药机制的重要靶点之一[8]. 除了诱导DNA损伤, GEM还以剂量依赖性和细胞依赖性的方式并入RNA, 并抑制RNA的合成, 尽管GEM的敏感性已证实与RNA结合量的差异相关, 但其具体机制仍不清楚[38]. 由于dFdCTP可能与CTP竞争结合RNA, 高浓度dFdCTP对CTP合成酶的抑制可能是dFdCTP结合RNA增加和RNA合成抑制的主要原因[8].
尽管GEM的细胞毒性作用与掺入DNA和RNA的药物量显著相关, 但其导致细胞死亡的下游分子途径仍未完全阐明[8]. 凋亡是包括GEM在内的细胞毒性药物引发细胞死亡的主要方式, 是细胞调节性死亡(regulated cell death, RCD)的一种, GEM所致的DNA损伤主要诱导内源性凋亡(又称"线粒体凋亡")[39]. 内源性凋亡的激活需要线粒体外膜通透性(mitochondrial outer membrane permeabilization, MOMP)的改变, 这种改变导致线粒体促凋亡蛋白如细胞色素C等的释放, 以触发凋亡小体的形成并激活下游凋亡启动子caspase-9和效应分子caspase-3, 引发一系列的凋亡信号级联反应. 其中, MOMP受BCL-2基因家族的严格控制, 该家族包括促凋亡(BAX、BAK等)和抗凋亡(BCL-2, BCL-xL和MCL-1等)成员[40]. 研究证实, 抗凋亡基因BCL-xL、MCL-1表达在对GEM获得性耐药的PDAC细胞中明显上调, 而促凋亡基因BAX、BAK表达则显著下调, 反复GEM暴露后上调BCL-xL、MCL-1增强了其对胰腺癌细胞的耐药性, 且BAX/BCL-2比值可预测GEM的敏感性[41], BCL-xL表达增强与PDAC患者生存期缩短相关[42]. 因此, BCL-2家族促凋亡和抗凋亡基因成员之间的平衡一定程度上决定了胰腺癌细胞对GEM的敏感性[43]. 此外, 促凋亡蛋白必须由唯BH3(BH3-only)蛋白激活后才能启动MOMP改变. 除了激活促凋亡蛋白, 单结构域的唯BH3蛋白家族(BID、BIM等)还可抑制抗凋亡蛋白, 共同促进细胞凋亡发生. 最新的一项研究显示, 上调唯BH3蛋白BIM可增加了PDAC细胞中GEM诱导的细胞死亡[44]. 因此, 靶向唯BH3蛋白表达是提高GEM化疗敏感性的新策略[45]. 当然, 内源性凋亡下游级联信号的激活还涉及多种caspase(如caspase-9/-3/-7等)关键酶, 这表明caspase也是评估GEM耐药性的重要标志物[46]. 最后, 抑癌基因p53也与GEM的化疗敏感性有关, 这依赖于p53蛋白对基因组完整性的维持和对凋亡的调控作用[47]. 研究显示, p53在GEM处理的野生型p53细胞系中明显上调, 且细胞中的BCL-2、BCL-xL蛋白水平降低, BAX蛋白水平升高. 然而, 突变型(失活)p53细胞中BCL-2水平则没有明显变化[48]. 因此, 野生型p53通过促进了凋亡增加了GEM的化疗敏感性, 而失活的p53则可能是通过激活促增殖JAK2-STAT3信号通路诱导了GEM耐药[49].
近年来, 伴随着自噬依赖性死亡、铁死亡(Ferroptosis)等新的RCD形式不断被发现[39], 其在化疗耐药中的作用也逐渐受到重视, 并为克服癌症耐药性研究提供了新的方向. 自噬是一种受控的分解代谢过程. 一方面, 自噬可以消除功能失调的蛋白质和细胞器, 保护癌细胞免受化疗药的毒性损伤; 另一方面, 在细胞内质网应激情况下, 自噬还会触发自噬依赖性细胞死亡, 从而逆转癌细胞的耐药性[50,51]. 因此, 肿瘤自噬在化疗耐药性中的作用可能是双向的. 体外和体内的研究均证实, 自噬主要是通过抑制PDAC细胞凋亡来实现对GEM的抵抗作用, 抑制自噬可以增强GEM的化疗敏感性[52]. 最新一项II期临床试验(NCT01506973)结果表明, 自噬抑制剂硫酸羟氯喹(HCQ)联合GEM+紫杉醇治疗PDAC可明显提高总体缓解率, 但遗憾的是未能改善1年总体生存率[53]. 另一项关于自噬相关蛋白VMP1(vacuole membrane protein 1)的研究显示, GEM可通过内质网应激诱导PDAC细胞VMP1表达并与对其耐药相关, 基于RNAi (RNA interference)技术的VMP1的基因失活可对抗这种耐药反应, 这一结果提示VMP1可作为对抗GEM耐药的潜在治疗靶点[54]. 至于PDAC中自噬依赖性细胞死亡与GEM耐药之间的关系, 目前仍不清楚. 其中, 自噬相关基因Beclin-1介导的自噬依赖性细胞死亡可能通过诱导凋亡来增强对GEM的敏感性, 而ROS/Akt/mTOR信号轴则可能参与抵抗这一作用[55]. 另外, Ferroptosis也是一种新定义的铁依赖性非凋亡细胞死亡形式, 与Kras基因突变密切相关, 并以细胞内铁的积累和随后的脂质过氧化为特征[56]. Kras基因是PDAC中最常见的突变基因, 因此靶向Ferroptosis作为一种胰腺癌治疗的新策略备受关注, 但Ferroptosis与GEM作用之间关系的研究仍十分有限[57]. 最近的一项研究显示, Kras/ERK信号传导途径下游的ARF6蛋白可通过降低RSL3(一种Ferroptosis的诱导分子)诱导Ferroptosis的敏感性, 抑制Ferroptosis通路, 并下调dCK和hENT1表达, 增强GEM的耐药性[58]. 因此, Ferroptosis通路的抑制也可能参与了PDAC对GEM的耐药过程.
通过对GEM敏感与耐药的PDAC细胞系间的能量代谢谱分析发现, 两者之间存在明显差异, 这提示GEM耐药可能与葡萄糖、氨基酸和脂质的代谢密切相关[59]. 因此, PDAC的代谢特征研究能为抗GEM耐药治疗提供新的策略.
乏氧和有氧糖酵解(Warburg效应)是PDAC细胞能量代谢的重要特征[60]. 研究表明, 诱导GEM耐药的PDAC细胞中出现有氧糖酵解增加和活性氧成分(reactive oxygen species, ROS)降低, 这一代谢转化是由缺氧诱导因子HIF-1α通过诱导参与糖酵解途径的酶和过表达葡萄糖转运蛋白(glucose transporter, GLUT)介导的. 糖酵解通过下调ROS水平进一步诱导并维持肿瘤干细胞(cancer stem cell, CSC)和上皮-间充质转化(epithelial-mesenchymal transition, EMT)表型, 增强对GEM的化疗抵抗[61]. 因此, 靶向调控细胞内ROS水平是GEM增敏的新策略, 且这一过程可能涉及Ferroptosis[62]. 此外, HIF-1α还可通过增加糖酵解促进DNA合成必需的非氧化磷酸戊糖途径(pentose phosphate pathway, PPP)及嘧啶生成, 导致细胞dCTP池增加, 从而减少dFdC并入DNA, 减弱GEM的细胞毒性. 除了缺氧, 跨膜黏蛋白MUC1的表达增加也能激活和稳定HIF-1α, 产生类似的作用. 因此, 抑制MUC1、HIF-1α、PPP或嘧啶合成均可增敏GEM[63]. 有趣的是, 最近一项研究显示, PDAC核苷转运体hENT1过表达也可以通过HIF-1α抑制葡萄糖转运和糖酵解, 逆转GEM耐药. 这为18F-FDG PET评估靶向hENT1逆转GEM耐药的疗效提供了可能[64]. 果糖-1,6-二磷酸酶(fructose-1, 6-bisphosphatase 1, FBP1)是PDAC糖异生过程中的关键酶之一, 对有氧糖酵解有负调节作用. 泛素特异性蛋白酶44(ubiquitin specific peptidase 44, USP44)可促进FBP1去泛素化以增加胰腺癌中FBP1蛋白的表达, USP44在PDAC细胞中被下调, 介导了糖酵解增加与GEM耐药[65]. 含F-box/WD重复序列的蛋白质7(F-box and WD repeat Domain containing 7, FBW7)是一种可被癌基因KRAS突变抑制的PDAC抑制因子, FBW7以c-Myc依赖性方式调节硫氧还蛋白互作蛋白(thioredoxin-interacting protein, TXNIP)表达, 后者能抑制糖酵解并增强GEM在异种移植模型中的疗效. 但直接靶向KRAS或c-Myc的治疗策略目前在技术上是困难的, 因此, 干扰c-Myc介导的下游效应分子有望成为克服GEM耐药的治疗新途径[66]. 此外, 胰腺癌细胞较邻近的正常组织表达更多的烟酰胺磷酸核糖转移酶(nicotinamide phosphoribosyltransferase, Nampt), Nampt是参与糖酵解氧化还原反应的烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide, NAD)生物合成的关键限速酶. 过表达的Nampt提供大量NAD, 增加PDAC细胞糖酵解, 有助于GEM抵抗, 而抑制Nampt则可逆转这种抵抗[67]. 在胰腺癌细胞中, TP53诱导的糖酵解和凋亡的调控子(TP53-induced glycolysis and apoptosis regulator gene, TIGAR)表达增加并促进其生存, TIGAR是野生型TP53的下游基因, 突变型TP53可以干扰TIGAR表达, 促进糖酵解和ROS相关的自噬[68]. 因此, TIGAR表达干扰也可能是突变型TP53所致GEM耐药的原因之一.
有关氨基酸代谢变化在GEM化疗耐药中作用的研究仍十分有限. 在各种氨基酸中谷氨酰胺(glutamine, Gln)是氮和碳的主要来源, 对癌细胞的生存至关重要. PDAC细胞中Gln摄取和分解增加, Gln代谢增强促进葡萄糖代谢的另一个分支(为糖基化提供底物)己糖胺生物合成途径(hexosamine biosynthesis pathway, HBP)和糖基化. 与生物合成机制有关的蛋白糖基化异常是PDAC的重要特征[69]. 其中, N-糖基化活性的提高可能与TGF-β、TNF和NF-κB等几种信号通路有关, 并且抑制N-糖基化可以显著改善胰腺癌细胞的耐药性[70]. 另外, L型氨基酸转运体2(L-type amino acid transporter-2, LAT2)是一种中性氨基酸转运蛋白, 也是PDAC中的一种致癌蛋白, 可以激活Gln依赖的mTOR信号通路, 抑制细胞凋亡并促进糖酵解, 导致对GEM耐药, 而抑制mTOR可以逆转这一过程[71]. 因此, 干扰Gln代谢途径是抵抗GEM耐药的新靶点[72]. PDAC对蛋氨酸(methionine, Met)的需求增加, 又被称为Met依赖. 干扰Met可选择性在细胞周期的S/G2期阻滞癌细胞, 促使癌细胞对化疗敏感. Met主要来自食物, 但通过低蛋白饮食限制Met的摄入十分困难. 最近一项研究显示, 用重组蛋氨酸酶(recombinant methioninase, rMETase)靶向干扰PDAC细胞Met代谢可实现对GEM的增敏. 由于癌组织吸收的Met远多于周围的正常组织, 因此可通过11C-MET-PET成像提供显著的Met信号评估靶向Met治疗的疗效[73]. 此外, 丝氨酸(serine, Ser)的生物合成也可能是PDAC细胞化疗耐药的组成部分. Ser对癌细胞增殖过程中的核苷酸和氨基酸合成至关重要, 有助于DNA损伤的修复. 诱导GEM耐药的PDAC细胞可通过MAPK级联反应上调Ser生物合成酶的表达, 促进Ser合成. BRAF 抑制剂(维罗非尼等)可抑制MAPK级联反应, 提高对GEM的敏感性[74].
脂质代谢也是癌症进展的必要条件之一, 其不仅为胞膜的形成提供了充分的物质基础, 而且为蛋白质的翻译后修饰提供了信号分子和底物[75]. 在PDAC中, 参与从头合成脂肪酸、胆固醇途径的许多酶如脂肪酸合成酶(fatty acid synthase, FASN)等明显上调, FASN是一种多功能蛋白同型二聚体, 可以将乙酰辅酶A和丙二酰辅酶A转化为棕榈酸酯, 从而调节脂质生成[76]. FASN的表达由转录因子固醇调节元件结合蛋白1c(sterol regulatory element-binding protein-1c, SREBP1c)调控, 后者是PI3K/Akt、MEK/ERK等信号通路的下游[75]. 其中, 高表达的FASN可上调丙酮酸激酶M2(pyruvate kinase M2, PKM2)的表达和P53信号通路, 促进糖酵解和抑制凋亡, 引发GEM抵抗[77,78]. 此外, 高表达的FASN还可通过减轻内质网应激, 维持CSC表型, 抑制GEM诱导的细胞凋亡. 一项研究亦证实, 在PDAC原位动物模型中抑制FASN可增加GEM的敏感性[79]. 不同的脂肪酸也在PDAC细胞的生长过程中发挥重要作用, 但具体机制仍待研究[6]. ω3不饱和脂肪酸(omega-3 polyunsaturated fatty acids, ω3-PUFA)可通过抑制促生存的NF-κB和Akt/STAT3通路活化, 增强GEM的抗癌作用[80]. 一项已完成的II期临床试验(NCT0109382)显示, GEM联合静脉ω3-PUFA治疗, 转移性或局部晚期PDAC患者的促炎循环生长因子和细胞因子减少, 并有助于改善预后[81]. 除脂肪酸外, 胆固醇代谢也参与了PDAC的进展. 胆固醇含量变化介导了如ERK1/2、PI3K/Akt、EGFR依赖的促生存途径的信号转导. 在异种小鼠模型中, 用shRNA (small hairpin RNA)沉默低密度脂蛋白受体(low density lipoprotein receptor, LDLR)可抑制胆固醇摄取, 增强GEM的细胞毒性[82]. 他汀类药物是胆固醇从头合成途径的抑制剂, 现已证实可提高PDAC患者的生存率, 但与GEM耐药间的关系仍待明确[83]. 一项针对晚期PDAC的Ⅱ期临床试验显示, 辛伐他汀联合GEM并未能提高GEM的疗效(NCT00944463)[84]. 总之, 越来越多的证据证实, PDAC细胞脂质代谢与GEM耐药之间存在密切关系, 抑制脂质代谢有助于克服耐药[6,85].
EMT是PDAC等上皮癌的细胞表型由上皮型向更具侵袭性的间充质型转化的动态过程[86]. 越来越多的证据表明, EMT与PDAC细胞的GEM化疗抵抗密切相关[87,88]. EMT通过上皮标记物(如E-钙黏蛋白、细胞角蛋白等)的下调和细胞间充质标志物(如波形蛋白、N-钙黏蛋白、纤连蛋白等)的上调, 造成细胞间失接触(封闭蛋白下调), 使癌细胞获得侵袭和迁移特性, 同时获得对GEM的天然抵抗力[89]. 这一结构和功能转化是通过TNF-α、TGF-β、HIF-1α、Wnt、Notch、RAS和NF-κB等介导的信号通路诱导间充质转录因子(Snail、Slug、ZEB-1、ZEB-2和Twist等)表达实现的, 但间充质转录因子下游的分子信号目前仍未明确[90,91]. E-钙黏蛋白的表达下调是EMT的表型标志, 这一过程受间充质转录因子的负性调控[90]. 基因表达谱分析表明, 细胞系对GEM的先天耐药性与E-钙黏蛋白的低表达和ZEB-1的高表达有关. 与先天耐药机制不同, 最近一项研究显示, PDAC的获得性耐药仅出现在GEM诱导的间充质样细胞中, 且这一过程由ERK/ZEB-1途径介导, 此时抑制ERK1/2磷酸化或ZEB-1表达可增敏GEM, 但预先处理则不会产生类似的效果[88]. 这或许是MEK/ERK抑制剂(曲美替尼等)联合GEM治疗晚期PDAC临床试验(NCT01231581、NCT01016483)失败的原因之一[92,93]. 总之, EMT状态变化与GEM耐药性密切相关, 靶向抑制EMT是PDAC克服GEM耐药的新策略.
近年来, 非编码RNA(non-coding RNA, ncRNA)包括微小RNA(microRNA, miRNA)和长非编码RNA(long non-coding RNAs, lncRNA)已被证实在PDAC的发生、发展中发挥重要作用[94]. miRNA是一类由19-25个核苷酸组成的非编码小RNA, 是一种基因表达的转录后调节因子. miRNA通过与目标mRNA的3'端非翻译区(UTR)互补结合, 并促进目标mRNA沉默或降解, 抑制蛋白质翻译, 实现对相关基因表达的调控[95]. 越来越多证据显示, miRNA失调(如miRNA-410-3p、miRNA-146a-5p等)可通过不同的促生存信号通路(自噬、NF-κB等)介导GEM抵抗[96,97]. 其中, miRNA靶向EMT途径关键蛋白的mRNA并促进EMT可能也参与了这一过程[98]. 此外, lncRNA也可促进PDAC对GEM的抵抗, 但具体机制仍不清楚[99]. 部分lncRNA和最近发现的特殊类型环状RNA(circular RNA, circRNA)可通过与miRNA结合并竞争性隔离其生理靶标, 从而负性调控miRNA的活性, 这类lncRNA又被称为"miRNA海绵"[94]. 因此, lncRNA作为miRNA海绵对miRNA活性调控作用可能是GEM化疗抵抗的原因之一[99,100]. 总之, ncRNA参与了PDAC对GEM的化疗抵抗, 靶向miRNA和miRNA海绵已成为研究新热点, 并终将为改善GEM耐药提供新的策略[101].
自GEM首次报道以来的20余年中, 其单独或联合应用已成为PDAC治疗最常用的一线治疗方案. 尽管最新的临床试验确立了其他如FOLFIRINOX等新的一线治疗选择, 但毒副作用的增加限制了其临床应用. 因此, GEM仍是晚期PDAC姑息治疗的基石. 对GEM的化疗耐药是影响PDAC治疗效果的决定性因素. 随着分子生物学的持续进展, 新的参与GEM抵抗的细胞内分子机制不断被揭示, 这些研究有助于为化疗增敏策略的开发提供新方向(图2, 表1). 然而, 目前一些针对肿瘤相关分子途径的治疗并没有取得令人满意的结果, 部分原因是替代代偿途径的快速上调, 而且肿瘤的异质性和体内、体外试验的差异更增加了其复杂程度, 这可能是部分临床试验失败的原因之一. 因此, 按异质性分层PDAC并开发更精准的GEM增敏方案将是未来研究发展的重点.
学科分类: 胃肠病学和肝病学
手稿来源地: 北京市
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B
C级 (良好): C, C
D级 (一般): D
E级 (差): 0
科学编辑: 马亚娟 制作编辑:张砚梁
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [PubMed] [DOI] |
| 2. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [PubMed] [DOI] |
| 3. | Pusceddu S, Ghidini M, Torchio M, Corti F, Tomasello G, Niger M, Prinzi N, Nichetti F, Coinu A, Di Bartolomeo M, Cabiddu M, Passalacqua R, de Braud F, Petrelli F. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019;11. [PubMed] [DOI] |
| 4. | Saif MW, Lee Y, Kim R. Harnessing gemcitabine metabolism: a step towards personalized medicine for pancreatic cancer. Ther. Adv Med Oncol. 2012;4:341-346. [PubMed] [DOI] |
| 5. | Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284-4292. [PubMed] [DOI] |
| 6. | Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, Zhao F, You L, Wang W, Zhao Y. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50. [PubMed] [DOI] |
| 7. | Rauchwerger DR, Firby PS, Hedley DW, Moore MJ. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000;60:6075-6079. [PubMed] |
| 8. | Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17 Suppl 5:v7-12. [PubMed] [DOI] |
| 9. | Elander NO, Aughton K, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Campbell F, Costello E, Halloran CM, Mackey JR, Scarfe AG, Valle JW, McDonald AC, Carter R, Tebbutt NC, Goldstein D, Shannon J, Dervenis C, Glimelius B, Deakin M, Charnley RM, Anthoney A, Lerch MM, Mayerle J, Oláh A, Büchler MW, Greenhalf W; European Study Group for Pancreatic Cancer. Expression of dihydropyrimidine dehydrogenase (DPD) and hENT1 predicts survival in pancreatic cancer. Br J Cancer. 2018;118:947-954. [PubMed] [DOI] |
| 10. | Raffenne J, Nicolle R, Puleo F, Le Corre D, Boyez C, Marechal R, Emile JF, Demetter P, Bardier A, Laurent-Puig P, de Mestier L, Paradis V, Couvelard A, VanLathem JL, MacKey JR, Bachet JB, Svrcek M, Cros J. hENT1 Testing in Pancreatic Ductal Adenocarcinoma: Are We Ready? A Multimodal Evaluation of hENT1 Status. Cancers (Basel). 2019;11. [PubMed] [DOI] |
| 11. | Bhutia YD, Hung SW, Patel B, Lovin D, Govindarajan R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Res. 2011;71:1825-1835. [PubMed] [DOI] |
| 12. | Maréchal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, Cass CE, Young J, Salmon I, Devière J, Van Laethem JL. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913-2919. [PubMed] [DOI] |
| 13. | Okamura Y, Yasukawa S, Narimatsu H, Boku N, Fukutomi A, Konishi M, Morinaga S, Toyama H, Kaneoka Y, Shimizu Y, Nakamori S, Sata N, Yamakita K, Takahashi A, Kainuma O, Hishinuma S, Yamaguchi R, Nagino M, Hirano S, Yanagisawa A, Mori K, Uesaka K. Human equilibrative nucleoside transporter-1 expression is a predictor in patients with resected pancreatic cancer treated with adjuvant S-1 chemotherapy. Cancer Sci. 2020;111:548-560. [PubMed] [DOI] |
| 14. | Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters-A review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7-30. [PubMed] [DOI] |
| 15. | Hioki M, Shimada T, Yuan T, Nakanishi T, Tajima H, Yamazaki M, Yokono R, Takabayashi M, Sawamoto K, Akashita G, Miyamoto KI, Ohta T, Tamai I, Shimada T, Sai Y. Contribution of equilibrative nucleoside transporters 1 and 2 to gemcitabine uptake in pancreatic cancer cells. Biopharm Drug Dispos. 2018;39:256-264. [PubMed] [DOI] |
| 16. | Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Büchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033-4040. [PubMed] |
| 17. | Skrypek N, Duchêne B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714-1723. [PubMed] [DOI] |
| 18. | Arana MR, Altenberg GA. ATP-binding Cassette Exporters: Structure and Mechanism with a Focus on P-glycoprotein and MRP1. Curr Med Chem. 2019;26:1062-1078. [PubMed] [DOI] |
| 19. | Kohan HG, Boroujerdi M. Time and concentration dependency of P-gp, MRP1 and MRP5 induction in response to gemcitabine uptake in Capan-2 pancreatic cancer cells. Xenobiotica. 2015;45:642-652. [PubMed] [DOI] |
| 20. | Ranjbar S, Khonkarn R, Moreno A, Baubichon-Cortay H, Miri R, Khoshneviszadeh M, Saso L, Edraki N, Falson P, Firuzi O. 5-Oxo-hexahydroquinoline derivatives as modulators of P-gp, MRP1 and BCRP transporters to overcome multidrug resistance in cancer cells. Toxicol Appl Pharmacol. 2019;362:136-149. [PubMed] [DOI] |
| 21. | Sampson A, Peterson BG, Tan KW, Iram SH. Doxorubicin as a fluorescent reporter identifies novel MRP1 (ABCC1) inhibitors missed by calcein-based high content screening of anticancer agents. Biomed Pharmacother. 2019;118:109289. [PubMed] [DOI] |
| 22. | Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110-6117. [PubMed] |
| 23. | Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3-10. [PubMed] |
| 24. | Ohmine K, Kawaguchi K, Ohtsuki S, Motoi F, Ohtsuka H, Kamiie J, Abe T, Unno M, Terasaki T. Quantitative Targeted Proteomics of Pancreatic Cancer: Deoxycytidine Kinase Protein Level Correlates to Progression-Free Survival of Patients Receiving Gemcitabine Treatment. Mol Pharm. 2015;12:3282-3291. [PubMed] [DOI] |
| 25. | Saiki Y, Yoshino Y, Fujimura H, Manabe T, Kudo Y, Shimada M, Mano N, Nakano T, Lee Y, Shimizu S, Oba S, Fujiwara S, Shimizu H, Chen N, Nezhad ZK, Jin G, Fukushige S, Sunamura M, Ishida M, Motoi F, Egawa S, Unno M, Horii A. DCK is frequently inactivated in acquired gemcitabine-resistant human cancer cells. Biochem Biophys Res Commun. 2012;421:98-104. [PubMed] [DOI] |
| 26. | Sierzega M, Pach R, Kulig P, Legutko J, Kulig J. Prognostic Implications of Expression Profiling for Gemcitabine-Related Genes (hENT1, dCK, RRM1, RRM2) in Patients With Resectable Pancreatic Adenocarcinoma Receiving Adjuvant Chemotherapy. Pancreas. 2017;46:684-689. [PubMed] [DOI] |
| 27. | Han Z, Li Y, Zhang J, Liu J, Chen C, van Zijl PC, Liu G. Molecular Imaging of Deoxycytidine Kinase Activity Using Deoxycytidine-Enhanced CEST MRI. Cancer Res. 2019;79:2775-2783. [PubMed] [DOI] |
| 28. | Wang C, Zhang W, Fu M, Yang A, Huang H, Xie J. Establishment of human pancreatic cancer gemcitabineresistant cell line with ribonucleotide reductase overexpression. Oncol Rep. 2015;33:383-390. [PubMed] [DOI] |
| 29. | Han QL, Zhou YH, Lyu Y, Yan H, Dai GH. Effect of ribonucleotide reductase M1 expression on overall survival in patients with pancreatic cancer receiving gemcitabine chemotherapy: A literature-based meta-analysis. J Clin Pharm Ther. 2018;43:163-169. [PubMed] [DOI] |
| 30. | Zhou J, Zhang L, Zheng H, Ge W, Huang Y, Yan Y, Zhou X, Zhu W, Kong Y, Ding Y, Wang W. Identification of chemoresistance-related mRNAs based on gemcitabine-resistant pancreatic cancer cell lines. Cancer Med. 2020;9:1115-1130. [PubMed] [DOI] |
| 31. | Frances A, Cordelier P. The Emerging Role of Cytidine Deaminase in Human Diseases: A New Opportunity for Therapy? Mol Ther. 2020;28:357-366. [PubMed] [DOI] |
| 32. | Mameri H, Bièche I, Meseure D, Marangoni E, Buhagiar-Labarchède G, Nicolas A, Vacher S, Onclercq-Delic R, Rajapakse V, Varma S, Reinhold WC, Pommier Y, Amor-Guéret M. Cytidine Deaminase Deficiency Reveals New Therapeutic Opportunities against Cancer. Clin Cancer Res. 2017;23:2116-2126. [PubMed] [DOI] |
| 33. | Rajabpour A, Afgar A, Mahmoodzadeh H, Radfar JE, Rajaei F, Teimoori-Toolabi L. MiR-608 regulating the expression of ribonucleotide reductase M1 and cytidine deaminase is repressed through induced gemcitabine chemoresistance in pancreatic cancer cells. Cancer Chemother Pharmacol. 2017;80:765-775. [PubMed] [DOI] |
| 34. | Ciccolini J, Dahan L, André N, Evrard A, Duluc M, Blesius A, Yang C, Giacometti S, Brunet C, Raynal C, Ortiz A, Frances N, Iliadis A, Duffaud F, Seitz JF, Mercier C. Cytidine deaminase residual activity in serum is a predictive marker of early severe toxicities in adults after gemcitabine-based chemotherapies. J Clin Oncol. 2010;28:160-165. [PubMed] [DOI] |
| 35. | Binenbaum Y, Na'ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2015;23:55-68. [PubMed] [DOI] |
| 36. | Akita H, Zheng Z, Takeda Y, Kim C, Kittaka N, Kobayashi S, Marubashi S, Takemasa I, Nagano H, Dono K, Nakamori S, Monden M, Mori M, Doki Y, Bepler G. Significance of RRM1 and ERCC1 expression in resectable pancreatic adenocarcinoma. Oncogene. 2009;28:2903-2909. [PubMed] [DOI] |
| 37. | Pourquier P, Gioffre C, Kohlhagen G, Urasaki Y, Goldwasser F, Hertel LW, Yu S, Pon RT, Gmeiner WH, Pommier Y. Gemcitabine (2',2'-difluoro-2'-deoxycytidine), an antimetabolite that poisons topoisomerase I. Clin Cancer Res. 2002;8:2499-2504. [PubMed] |
| 38. | Kroep JR, Giaccone G, Tolis C, Voorn DA, Loves WJ, Groeningen CJ, Pinedo HM, Peters GJ. Sequence dependent effect of paclitaxel on gemcitabine metabolism in relation to cell cycle and cytotoxicity in non-small-cell lung cancer cell lines. Br J Cancer. 2000;83:1069-1076. [PubMed] [DOI] |
| 39. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [PubMed] [DOI] |
| 40. | Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310:66-67. [PubMed] [DOI] |
| 41. | Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354-362. [PubMed] [DOI] |
| 42. | Friess H, Lu Z, Andrén-Sandberg A, Berberat P, Zimmermann A, Adler G, Schmid R, Büchler MW. Moderate activation of the apoptosis inhibitor bcl-xL worsens the prognosis in pancreatic cancer. Ann Surg. 1998;228:780-787. [PubMed] [DOI] |
| 43. | Bauer C, Hees C, Sterzik A, Bauernfeind F, Mak'Anyengo R, Duewell P, Lehr HA, Noessner E, Wank R, Trauzold A, Endres S, Dauer M, Schnurr M. Proapoptotic and antiapoptotic proteins of the Bcl-2 family regulate sensitivity of pancreatic cancer cells toward gemcitabine and T-cell-mediated cytotoxicity. J Immunother. 2015;38:116-126. [PubMed] [DOI] |
| 44. | Suhaili SH, Karimian H, Stellato M, Lee TH, Aguilar MI. Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization. Biophys Rev. 2017;9:443-457. [PubMed] [DOI] |
| 45. | Xie F, Huang M, Lin X, Liu C, Liu Z, Meng F, Wang C, Huang Q. The BET inhibitor I-BET762 inhibits pancreatic ductal adenocarcinoma cell proliferation and enhances the therapeutic effect of gemcitabine. Sci Rep. 2018;8:8102. [PubMed] [DOI] |
| 46. | Lim CY, Chang JH, Lee WS, Lee KM, Yoon YC, Kim J, Park IY. Organotypic slice cultures of pancreatic ductal adenocarcinoma preserve the tumor microenvironment and provide a platform for drug response. Pancreatology. 2018;18:913-927. [PubMed] [DOI] |
| 47. | Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921-8946. [PubMed] [DOI] |
| 48. | Galmarini CM, Clarke ML, Falette N, Puisieux A, Mackey JR, Dumontet C. Expression of a non-functional p53 affects the sensitivity of cancer cells to gemcitabine. Int J Cancer. 2002;97:439-445. [PubMed] [DOI] |
| 49. | Wörmann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 2016;151:180-193.e12. [PubMed] [DOI] |
| 50. | Nassour J, Radford R, Correia A, Fusté JM, Schoell B, Jauch A, Shaw RJ, Karlseder J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659-663. [PubMed] [DOI] |
| 51. | Zhou Y, Shen Y, Chen C, Sui X, Yang J, Wang L, Zhou J. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Cancer Biol Med. 2019;16:630-646. [PubMed] [DOI] |
| 52. | Papademetrio DL, Cavaliere V, Simunovich T, Costantino S, Campos MD, Lombardo T, Kaiser CM, Alvarez E. Interplay between autophagy and apoptosis in pancreatic tumors in response to gemcitabine. Target Oncol. 2014;9:123-134. [PubMed] [DOI] |
| 53. | Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, De Jesus-Acosta A, Redlinger C, Burrell JA, Laheru DA, Von Hoff DD, Amaravadi RK, Drebin JA, O'Dwyer PJ. Effect of Gemcitabine and nab-Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:993-998. [PubMed] [DOI] |
| 54. | Gilabert M, Vaccaro MI, Fernandez-Zapico ME, Calvo EL, Turrini O, Secq V, Garcia S, Moutardier V, Lomberk G, Dusetti N, Urrutia R, Iovanna JL. Novel role of VMP1 as modifier of the pancreatic tumor cell response to chemotherapeutic drugs. J Cell Physiol. 2013;228:1834-1843. [PubMed] [DOI] |
| 55. | Fiorini C, Cordani M, Gotte G, Picone D, Donadelli M. Onconase induces autophagy sensitizing pancreatic cancer cells to gemcitabine and activates Akt/mTOR pathway in a ROS-dependent manner. Biochim Biophys Acta. 2015;1853:549-560. [PubMed] [DOI] |
| 56. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [PubMed] [DOI] |
| 57. | Chen G, Guo G, Zhou X, Chen H. Potential mechanism of ferroptosis in pancreatic cancer. Oncol Lett. 2020;19:579-587. [PubMed] [DOI] |
| 58. | Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M, Sun Q, Zhang Z, Liu W, Xu W, Ji S, Yu X, Xu X, Qin Y. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res. 2020;10:1182-1193. [PubMed] |
| 59. | Fujimura Y, Ikenaga N, Ohuchida K, Setoyama D, Irie M, Miura D, Wariishi H, Murata M, Mizumoto K, Hashizume M, Tanaka M. Mass spectrometry-based metabolic profiling of gemcitabine-sensitive and gemcitabine-resistant pancreatic cancer cells. Pancreas. 2014;43:311-318. [PubMed] [DOI] |
| 60. | Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919-922. [PubMed] [DOI] |
| 61. | Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen Q, Wang C, Yin T. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21:2055-2067. [PubMed] [DOI] |
| 62. | Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med. 2017;104:144-164. [PubMed] [DOI] |
| 63. | Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, Illies AL, Gebregiworgis T, Dai B, Augustine JJ, Murthy D, Attri KS, Mashadova O, Grandgenett PM, Powers R, Ly QP, Lazenby AJ, Grem JL, Yu F, Matés JM, Asara JM, Kim JW, Hankins JH, Weekes C, Hollingsworth MA, Serkova NJ, Sasson AR, Fleming JB, Oliveto JM, Lyssiotis CA, Cantley LC, Berim L, Singh PK. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell. 2017;32:71-87.e7. [PubMed] [DOI] |
| 64. | Xi Y, Yuan P, Li T, Zhang M, Liu MF, Li B. hENT1 reverses chemoresistance by regulating glycolysis in pancreatic cancer. Cancer Lett. 2020;479:112-122. [PubMed] [DOI] |
| 65. | Yang C, Zhu S, Yang H, Deng S, Fan P, Li M, Jin X. USP44 suppresses pancreatic cancer progression and overcomes gemcitabine resistance by deubiquitinating FBP1. Am J Cancer Res. 2019;9:1722-1733. [PubMed] |
| 66. | Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, Jin K, Liang D, Xu W, Zhang B, Liu L, Liu C, Xu J, Ni Q, Chiao PJ, Li M, Yu X. FBW7 (F-box and WD Repeat Domain-Containing 7) Negatively Regulates Glucose Metabolism by Targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) Axis in Pancreatic Cancer. Clin Cancer Res. 2016;22:3950-3960. [PubMed] [DOI] |
| 67. | Ju HQ, Zhuang ZN, Li H, Tian T, Lu YX, Fan XQ, Zhou HJ, Mo HY, Sheng H, Chiao PJ, Xu RH. Regulation of the Nampt-mediated NAD salvage pathway and its therapeutic implications in pancreatic cancer. Cancer Lett. 2016;379:1-11. [PubMed] [DOI] |
| 68. | Xie JM, Li B, Yu HP, Gao QG, Li W, Wu HR, Qin ZH. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74:5127-5138. [PubMed] [DOI] |
| 69. | Vasconcelos-Dos-Santos A, Oliveira IA, Lucena MC, Mantuano NR, Whelan SA, Dias WB, Todeschini AR. Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer. Front Oncol. 2015;5:138. [PubMed] [DOI] |
| 70. | Pan S, Chen R, Tamura Y, Crispin DA, Lai LA, May DH, McIntosh MW, Goodlett DR, Brentnall TA. Quantitative glycoproteomics analysis reveals changes in N-glycosylation level associated with pancreatic ductal adenocarcinoma. J Proteome Res. 2014;13:1293-1306. [PubMed] [DOI] |
| 71. | Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J, You L, Zheng L, Zhang T, Zhao Y. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274. [PubMed] [DOI] |
| 72. | Chen R, Lai LA, Sullivan Y, Wong M, Wang L, Riddell J, Jung L, Pillarisetty VG, Brentnall TA, Pan S. Disrupting glutamine metabolic pathways to sensitize gemcitabine-resistant pancreatic cancer. Sci Rep. 2017;7:7950. [PubMed] [DOI] |
| 73. | Kawaguchi K, Miyake K, Han Q, Li S, Tan Y, Igarashi K, Lwin TM, Higuchi T, Kiyuna T, Miyake M, Oshiro H, Bouvet M, Unno M, Hoffman RM. Targeting altered cancer methionine metabolism with recombinant methioninase (rMETase) overcomes partial gemcitabine-resistance and regresses a patient-derived orthotopic xenograft (PDOX) nude mouse model of pancreatic cancer. Cell Cycle. 2018;17:868-873. [PubMed] [DOI] |
| 74. | Ross KC, Andrews AJ, Marion CD, Yen TJ, Bhattacharjee V. Identification of the Serine Biosynthesis Pathway as a Critical Component of BRAF Inhibitor Resistance of Melanoma, Pancreatic, and Non-Small Cell Lung Cancer Cells. Mol Cancer Ther. 2017;16:1596-1609. [PubMed] [DOI] |
| 75. | Sunami Y, Rebelo A, Kleeff J. Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells. Cancers (Basel). 2017;10. [PubMed] [DOI] |
| 76. | Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, Klein A, Griffith M, Omura N, Medghalchi S, Kuhajda F, Goggins M. Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev. 2009;18:2380-2385. [PubMed] [DOI] |
| 77. | Tian S, Li P, Sheng S, Jin X. Upregulation of pyruvate kinase M2 expression by fatty acid synthase contributes to gemcitabine resistance in pancreatic cancer. Oncol Lett. 2018;15:2211-2217. [PubMed] [DOI] |
| 78. | Kim DJ, Park YS, Kang MG, You YM, Jung Y, Koo H, Kim JA, Kim MJ, Hong SM, Lee KB, Jang JJ, Park KC, Yeom YI. Pyruvate kinase isoenzyme M2 is a therapeutic target of gemcitabine-resistant pancreatic cancer cells. Exp Cell Res. 2015;336:119-129. [PubMed] [DOI] |
| 79. | Tadros S, Shukla SK, King RJ, Gunda V, Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, Grem J, Sasson AR, Singh PK. De Novo Lipid Synthesis Facilitates Gemcitabine Resistance through Endoplasmic Reticulum Stress in Pancreatic Cancer. Cancer Res. 2017;77:5503-5517. [PubMed] [DOI] |
| 80. | Hering J, Garrean S, Dekoj TR, Razzak A, Saied A, Trevino J, Babcock TA, Espat NJ. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann Surg Oncol. 2007;14:3620-3628. [PubMed] [DOI] |
| 81. | Arshad A, Chung WY, Steward W, Metcalfe MS, Dennison AR. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford). 2013;15:428-432. [PubMed] [DOI] |
| 82. | Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O, Pinault M, Guimaraes C, Nigri J, Loncle C, Lavaut MN, Garcia S, Tailleux A, Staels B, Calvo E, Tomasini R, Iovanna JL, Vasseur S. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:2473-2478. [PubMed] [DOI] |
| 83. | Huang BZ, Chang JI, Li E, Xiang AH, Wu BU. Influence of Statins and Cholesterol on Mortality Among Patients With Pancreatic Cancer. J Natl Cancer Inst. 2017;109. [PubMed] [DOI] |
| 84. | Hong JY, Nam EM, Lee J, Park JO, Lee SC, Song SY, Choi SH, Heo JS, Park SH, Lim HY, Kang WK, Park YS. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73:125-130. [PubMed] [DOI] |
| 85. | Shetty A, Nagesh PKB, Setua S, Hafeez BB, Jaggi M, Yallapu MM, Chauhan SC. Novel Paclitaxel Nanoformulation Impairs De Novo Lipid Synthesis in Pancreatic Cancer Cells and Enhances Gemcitabine Efficacy. ACS Omega. 2020;5:8982-8991. [PubMed] [DOI] |
| 86. | Ribatti D, Tamma R, Annese T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl Oncol. 2020;13:100773. [PubMed] [DOI] |
| 87. | Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525-530. [PubMed] [DOI] |
| 88. | El Amrani M, Corfiotti F, Corvaisier M, Vasseur R, Fulbert M, Skrzypczyk C, Deshorgues AC, Gnemmi V, Tulasne D, Lahdaoui F, Vincent A, Pruvot FR, Van Seuningen I, Huet G, Truant S. Gemcitabine-induced epithelial-mesenchymal transition-like changes sustain chemoresistance of pancreatic cancer cells of mesenchymal-like phenotype. Mol Carcinog. 2019;58:1985-1997. [PubMed] [DOI] |
| 89. | Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820-5828. [PubMed] [DOI] |
| 90. | Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488-494. [PubMed] [DOI] |
| 91. | Singh M, Yelle N, Venugopal C, Singh SK. EMT: Mechanisms and therapeutic implications. Pharmacol Ther. 2018;182:80-94. [PubMed] [DOI] |
| 92. | Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K, Le N. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072-2081. [PubMed] [DOI] |
| 93. | Van Cutsem E, Hidalgo M, Canon JL, Macarulla T, Bazin I, Poddubskaya E, Manojlovic N, Radenkovic D, Verslype C, Raymond E, Cubillo A, Schueler A, Zhao C, Hammel P. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer. 2018;143:2053-2064. [PubMed] [DOI] |
| 94. | Bak RO, Mikkelsen JG. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdiscip Rev RNA. 2014;5:317-333. [PubMed] [DOI] |
| 95. | Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [PubMed] [DOI] |
| 96. | Xiong J, Wang D, Wei A, Ke N, Wang Y, Tang J, He S, Hu W, Liu X. MicroRNA-410-3p attenuates gemcitabine resistance in pancreatic ductal adenocarcinoma by inhibiting HMGB1-mediated autophagy. Oncotarget. 2017;8:107500-107512. [PubMed] [DOI] |
| 97. | Meng Q, Liang C, Hua J, Zhang B, Liu J, Zhang Y, Wei M, Yu X, Xu J, Shi S. A miR-146a-5p/TRAF6/NF-kB p65 axis regulates pancreatic cancer chemoresistance: functional validation and clinical significance. Theranostics. 2020;10:3967-3979. [PubMed] [DOI] |
| 98. | Dhayat SA, Traeger MM, Rehkaemper J, Stroese AJ, Steinestel K, Wardelmann E, Kabar I, Senninger N. Clinical Impact of Epithelial-to-Mesenchymal Transition Regulating MicroRNAs in Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2018;10. [PubMed] [DOI] |
| 99. | Xiong G, Liu C, Yang G, Feng M, Xu J, Zhao F, You L, Zhou L, Zheng L, Hu Y, Wang X, Zhang T, Zhao Y. Long noncoding RNA GSTM3TV2 upregulates LAT2 and OLR1 by competitively sponging let-7 to promote gemcitabine resistance in pancreatic cancer. J Hematol Oncol. 2019;12:97. [PubMed] [DOI] |
| 100. | Hua YQ, Zhu YD, Xie GQ, Zhang K, Sheng J, Zhu ZF, Ning ZY, Chen H, Chen Z, Meng ZQ, Liu LM. Long non-coding SBF2-AS1 acting as a competing endogenous RNA to sponge microRNA-142-3p to participate in gemcitabine resistance in pancreatic cancer via upregulating TWF1. Aging (Albany NY). 2019;11:8860-8878. [PubMed] [DOI] |
| 101. | Liu Y, Xia L, Dong L, Wang J, Xiao Q, Yu X, Zhu H. CircHIPK3 Promotes Gemcitabine (GEM) Resistance in Pancreatic Cancer Cells by Sponging miR-330-5p and Targets RASSF1. Cancer Manag Res. 2020;12:921-929. [PubMed] [DOI] |