修回日期: 2018-10-23
接受日期: 2018-11-08
在线出版日期: 2019-02-08
慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)是我国及亚太地区慢性肝病患者常见的危重急症, 其发病机制尚未完全阐明, 且目前缺乏有效的治疗方法. 近年研究显示, 与发生在无慢性肝病基础上的急性肝衰竭患者相比, 有慢性肝病基础的患者抵抗后续急性打击的能力更强. 该发现促使临床专家和基础研究者从慢性肝病基础上损伤抵抗这一新的角度出发来探讨ACLF的发病机制. 本文就肝纤维化基础上损伤抵抗(模拟ACLF)现象及其细胞与分子机制的研究现状作一述评, 以期为阐明ACLF的发病机制及寻找新的治疗靶点提供理论依据.
核心提要: 纤维化肝脏中M2-样活化的巨噬细胞发挥着抵抗急性打击的有益肝保护效应, 其作用机制主要包括两个方面: (1)促进介导损伤效应的M1-样巨噬细胞凋亡, 抑制肝细胞凋亡; (2)抑制HMGB1的转位和释放及其介导的促炎免疫反应. 调控巨噬细胞的M1/M2活化有望成为控制慢加急性肝衰竭发展及影响疾病转归的重要策略.
引文著录: 白丽, 陈煜, 段钟平, 郑素军. 慢加急性肝衰竭研究的新视角: 肝纤维化与损伤抵抗. 世界华人消化杂志 2019; 27(3): 139-145
Revised: October 23, 2018
Accepted: November 8, 2018
Published online: February 8, 2019
Acute-on-chronic liver failure (ACLF) is an increasingly recognized entity encompassing an acute deterioration of liver function in patients with pre-existing chronic liver diseases, which is usually associated with a precipitating event. Compared to acute liver failure, ACLF patients exhibit relatively slow disease progression and prolonged survival. Recent studies show that patients without previous decompensation have higher short-term mortality than those with prior hepatic decompensation. These interesting and important facts motivate clinicians and researchers to dissect the underlying mechanisms of ACLF from a new perspective, namely, the correlation between chronic liver diseases and injury resistance. In this review, we will make a comment on the phenomena as well as cellular and molecular mechanisms behind injury resistance in the setting of hepatic fibrosis (simulating the development of ACLF), in hopes of providing novel insights into the pathogenesis and therapy of ACLF.
- Citation: Bai L, Chen Y, Duan ZP, Zheng SJ. A new perspective on acute-on-chronic liver failure: Liver fibrosis and injury resistance. Shijie Huaren Xiaohua Zazhi 2019; 27(3): 139-145
- URL: https://www.wjgnet.com/1009-3079/full/v27/i3/139.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v27.i3.139
慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)是在慢性肝病基础上出现肝功能急剧恶化的一种临床综合征. 它是我国及亚太地区慢性肝病患者常见的危重急症, 也是我国肝病患者死亡的重要原因之一. 尽管经过多年来的不断发展进步ACLF的临床治疗水平有所提高, 目前ACLF患者的死亡率依然居高不下, 如果不进行肝移植治疗, 其死亡率可高达50%以上, 给患者生命带来严重威胁, 同时也给家庭、社会造成沉重的经济负担. 迫切的临床和社会需求亟待我们从新的角度、更深层次探讨ACLF的发病机制, 以期获得能够提高临床疗效、延长患者生存时间、降低病死率的治疗新策略.
临床上, 急性肝衰竭(acute liver failure, ALF)无既往肝病史, 因遭受急性打击因素(例如病毒、酒精、药物等)的攻击而发病[1,2]; 而ACLF通常发生在慢性肝病(慢性肝炎、肝纤维化/肝硬化)基础上, 在遭受急性打击后肝病加重[3,4]. 人们很可能认为, 无肝病基础的ALF的病情要比ACLF更轻. 然而, 实际上, ALF起病急剧, 进展迅猛, 从黄疸进展至肝性脑病的间隔可短至2 wk, 死亡率极高[1,5]; 相较ALF, ACLF的肝损伤相对较轻, 疾病进展相对较缓慢, 死亡率也相对较低[6]. 这一有趣的临床现象引起了临床专家和基础研究者的关注. 最新报道显示, 既往有肝病失代偿发作的ACLF患者, 其病情和短期死亡率较既往无失代偿发作患者有所减轻/缓解. 有肝病失代偿既往史的患者在暴露于新的毒性刺激时, 其对肝损伤的抵抗性增强[6,7]. 也就是说, 该情况下的肝病失代偿并非一种有害事件, 而可能是一种有益的保护效应. 此外, ACLF患者在出现脓毒症和器官衰竭之前存在治疗的"黄金窗口期", 在该时间段给予适当的干预治疗, 患者的病情有可能完全逆转[7,8]. 这就给ACLF患者提供了宝贵的治疗机会. 而研究慢性肝病基础上损伤抵抗这一主题, 对于ACLF发病机制的阐明和治疗策略的制定具有重要的临床意义和研究价值.
鉴于目前还没有公认的ACLF模型, 研究者常采用肝纤维化基础上给予急性打击的动物模型来模拟ACLF的发病. 众所周知, 肝纤维化是各种慢性肝脏疾病的共同终末途径, 可进一步发展为肝硬化. 因此, 肝纤维化常被视为一种有害的病理反应. 然而, 近年来的有力证据使肝纤维化呈现出其作为"双刃剑"的另一面, 即其强大的伤口愈合反应所诱导产生的对后续急性肝损伤的有益保护作用. 肝纤维化可以由四氯化碳(carbon tetrachloride, CCl4)、硫代乙酰胺(thioacetamide, TAA)等药物或胆管结扎(bile duct ligation, BDL)诱导, 而急性打击因素包括D-氨基半乳糖/脂多糖(D-galactosaminide/lipopolysaccharide, D-GalN/LPS)[9]、对乙酰氨基酚(acetaminophen, APAP)[10]、CCl4[11]、TNF-α/Fas诱导的凋亡[12-14]等. 这些研究均证明, 肝纤维化能够赋予小鼠抵抗后续急性损伤的能力. 临床实践中, 肝酶慢性升高的患者对他汀类药物肝毒性的敏感性低于肝酶正常者[15]. 另外, 有慢性肝病基础的患者对APAP的耐受性较健康人更高[16]. 这些发现也支持慢性肝病基础上损伤抵抗的存在.
研究者进一步从细胞水平探讨了引起这种有益肝保护作用的机制. 鉴于炎症和免疫是肝衰竭发病的主要特征[7], 而巨噬细胞作为一种重要的天然免疫细胞, 在多种急、慢性肝损伤(包括肝衰竭)的启动、进展和恢复中均发挥着关键作用[17-20], 因而研究者将巨噬细胞作为探讨ACLF发病机制的重要靶细胞. 日本Osawa等[13]的研究显示, 耗竭肝脏驻留巨噬细胞(Kupffer细胞)后, 由胆管结扎所诱导的肝纤维化的肝脏保护效应丧失, 说明巨噬细胞在肝纤维化基础上的损伤抵抗中扮演着重要角色. 该课题组进一步证明, 巨噬细胞介导的这一肝保护效应是通过诱导AKT活化、促进肝细胞生存/肝再生来实现的. 我们的前期研究[11]显示, 敲除正常小鼠的巨噬细胞, 再给予急性攻击, 其肝损害较未敲除组明显减轻; 而耗竭肝纤维化小鼠的巨噬细胞, 再给予急性攻击, 其肝损害则明显加重, 且多种损伤标记的表达显著升高. 由此可见, 巨噬细胞在急慢性肝损伤中发挥着双重效应: 急性肝损伤中的巨噬细胞发挥着促进肝损害的效应, 而肝纤维化中的巨噬细胞发挥着保护小鼠抵抗急性打击的作用.
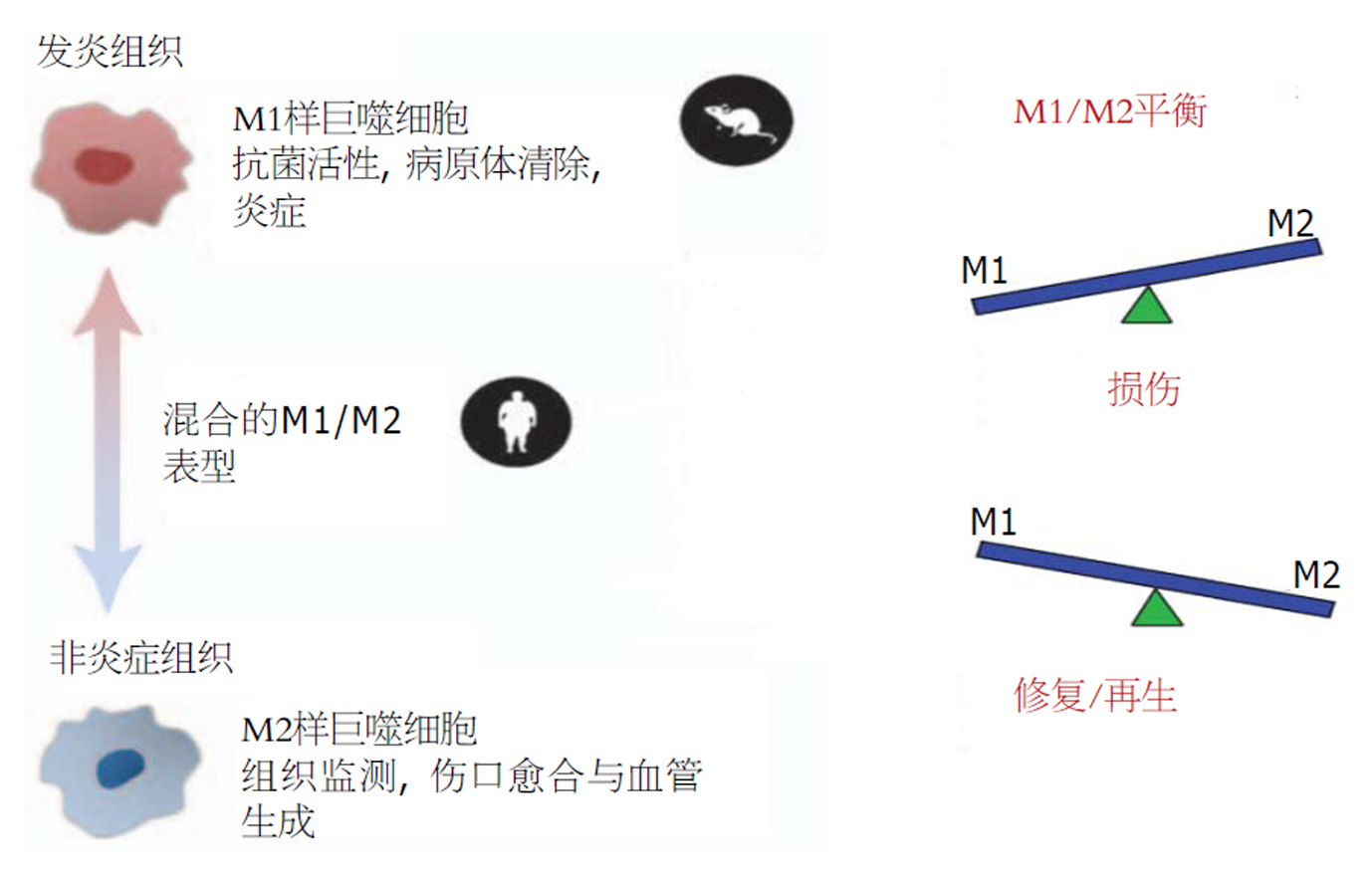
为什么巨噬细胞在急慢性肝损伤中会呈现出截然不同的功能呢?我们从新的角度, 即从巨噬细胞活化的角度对此进行了探讨. 巨噬细胞具有极强的可塑性和明显的异质性, 其所处微环境信号是决定其表型及功能的关键因素[21-23]. 根据传统分类方法, 巨噬细胞可分为两大类, 经典活化的(M1)巨噬细胞和替代活化的(M2)巨噬细胞. M1活化由脂多糖(LPS)或者Th1细胞因子(如IFN-γ)驱动, 活化的M1巨噬细胞可释放多种细胞因子和毒性介质, 从而引起炎症及组织损害. Th2细胞因子(如IL-4和IL-13)可促使巨噬细胞极化为M2表型. 与M1型巨噬细胞相反, M2极化的巨噬细胞可释放多种免疫调节介质, 从而发挥抗炎以及促进组织修复的功能. 需要注意的是, 巨噬细胞M1/M2极化只是巨噬细胞自身适应微环境的极端情况. 在肝脏疾病中, 巨噬细胞并不会呈现出单一的"促炎"M1或"免疫调节"M2表型. 相反, 体内巨噬细胞能够同时表达M1和M2型分化标志, 其表型为介于M1和M2活化之间的混合表型. 而M1/M2活化的平衡倾向决定了巨噬细胞呈现M1-样或M2-样表型并执行相应的功能[21-25](图1).
我们的前期研究[11]表明: (1)急性损伤时巨噬细胞呈现M1-为主的活化(iNOS、IL-1β等高表达), 而肝纤维化中巨噬细胞呈现M2-为主的活化(CD206、Arg-1、CCL17等高表达). 也就是说, 肝脏巨噬细胞相应于急性肝损伤或者纤维化环境, 分别活化为M1-样或M2-样表型. (2)如前所述, 巨噬细胞敲除的正常小鼠接受急性打击时, 其肝损害减轻, 也就是说, M1-样活化的巨噬细胞很可能介导了肝脏损伤效应; 巨噬细胞耗竭的纤维化小鼠接受急性打击时, 其肝损害明显加重, 这就说明, M2-样活化的巨噬细胞很可能介导了肝脏保护效应. (3)给正常小鼠回输从纤维化肝脏分离的巨噬细胞(M2-样), 然后给予急性攻击, 其损伤较未回输组明显减轻, 进一步证明了M2-样巨噬细胞的肝脏保护效应. 因此, 巨噬细胞的M2-样活化与肝纤维化基础上的损伤抵抗密切相关.
肝脏稳态的维持依赖于细胞生长、增殖和细胞死亡之间的平衡. 程序性细胞死亡(凋亡)作为一种主要的细胞死亡模式, 已经被证明在多种急慢性肝病中显著增强. Wan及其同事研究了替代活化的M2 Kupffer细胞(KC)对酒精和高脂诱导的肝损伤的影响, 他们揭示了M2 KC抵抗酒精性肝病(alcoholic liver disease, ALD)和非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)的新机制, 即M2 KC促进了介导肝损伤效应的M1 KC的凋亡[27]. Wan等[28]的另一项研究发现, 在体内和体外, M2极化的巨噬细胞可促进肝细胞衰老, 从而使肝细胞对酒精诱导凋亡的抵抗性增强. 我们最近的研究[11]显示, 急性损伤组小鼠的肝组织中存在广泛的凋亡, 这就意味着细胞增殖/凋亡的平衡倾向于细胞死亡; 而对于肝纤维化小鼠, 即使是在急性攻击情况下, 肝细胞的凋亡仍明显受抑, 表明该平衡很可能倾向于肝细胞生长/增殖. 最新体外研究显示[11,29], 源于M2活化巨噬细胞的条件性培养基能够保护人和小鼠肝细胞(原代小鼠肝细胞和人肝细胞系)抵抗TNF-α/D-GalN诱导的凋亡, 而源于M0或M1活化巨噬细胞的条件性培养基则对肝细胞凋亡无明显影响. 因此, M2-样巨噬细胞可促使细胞增殖/凋亡的平衡向细胞增殖倾斜, 而发挥肝保护作用.
高迁移率族蛋白1(high-mobility group box 1, HMGB1)是一种进化保守的非组蛋白DNA结合蛋白, 其功能的发挥取决于它在细胞内的定位: 核内的HMGB1作为一种DNA分子伴侣, 参与多种DNA活性相关事件, 如DNA复制、修复、重组、转录和遗传稳定性; 当细胞受到刺激时, HMGBl发生核浆转位, 可由死亡或损伤细胞被动释放或者由炎性免疫细胞主动分泌到胞外. 胞外HMGB1作为损伤相关分子模式(damage-associated molecular pattern, DAMP)蛋白与多种受体结合, 可以诱导机体固有免疫, 启动炎症反应[30,31]. HMGBl作为一种公认的危险信号分子, 已被证明参与多种肝病的病理过程, 包括肝纤维化和肝衰竭[32,33].
我们的前期研究显示, APAP可触发正常小鼠肝组织中HMGB1的显著高表达及明显的胞质转位; 而在纤维化肝组织中, 即使是在接受APAP攻击的情况下, HMGBl仍低表达, 且大多定位于胞核[10]. 值得注意的是, 停止给予CCl4后, 随着肝纤维化的消融, HMGB1的转位和释放逐渐增强. 纤维消融第12天时, 由急性打击所触发的HMGB1转位和释放水平与急性肝损伤组相近[34]. 体外研究显示, LPS或者HMGB1肽段可诱使从正常小鼠分离的M0巨噬细胞中HMGB1的广泛转位; 而对于从纤维化小鼠分离的M2-样巨噬细胞, 即使是在LPS或者HMGB1肽段的刺激下, 其HMGB1仍主要定位于胞核[35]. 因此, M2-样巨噬细胞能够抑制HMGB1的转位和释放. 鉴于HMGB1是一种强效促炎因子, M2-样巨噬细胞对HMGB1转位和释放的抑制, 可进一步引起对组织或细胞炎症反应的抑制, 从而减轻肝损害, 改善预后.
在体内, 巨噬细胞的表型并不是固定不变的, 而是随着微环境的改变出现促炎M1型和抗炎M2型之间的转换. 因此, 体内M1/M2活化之间微妙的平衡是维持稳态的重要因素. 一旦这种平衡被打破, 也就是说巨噬细胞呈现出M1-为主或M2为主的活化表型, 则可能促进急慢性损伤. 由于巨噬细胞活化的可塑性、动态性, 如何调控其活化及有针对性地改变其活化状态, 对于控制疾病发展和影响疾病转归具有重要意义[36].
巨噬细胞的活化受控于信号通路分子、转录因子及表观遗传学等多种因素[37,38]. 在众多的极化调控分子中, 信号转导及转录激活因子(signal transducers and activators of transcription, STATs)家族是一类最主要的调控分子. STATs家族有7个成员, 它们结构相似, 但功能不同, 从而维持着巨噬细胞M1/M2极化的平衡. 例如, 干扰素可活化STAT1, 促进M1极化; 而白细胞介素-10(interleukin-10, IL-10)则能通过活化STAT3促使巨噬细胞向M2方向极化. IL-10是一种多效性细胞因子, 在肝衰竭发病中发挥抗炎和免疫抑制作用. IL-10可由巨噬细胞、树突状细胞、T细胞等多种细胞产生. 分泌的IL-10细胞因子与膜表面的IL-10受体1(IL-10R1)结合, 再与IL-10R2二聚化, 进而募集胞浆蛋白Jak1, 随后使STAT3的705位酪氨酸磷酸化. 磷酸化的STAT3形成同聚物, 然后转位至胞核以促进靶基因的转录调节(上调Arg-1、IL-4Rα等, 抑制TNF-α、IL-6、IL-12、IL-1β). IL-10/STAT3信号通路已被证明为巨噬细胞极化至M2表型的决定性因素[39,40]. 我们在前期研究[11]中发现, 替代活化的巨噬细胞培养上清中IL-10表达明显上调. 给正常小鼠回输M2样巨噬细胞再给予急性打击, 其肝损伤较急性损伤组明显减轻, 外周血IL-10水平也明显升高, 提示IL-10信号可能与M2-样巨噬细胞的肝保护作用密切相关. 然而, IL-10/STAT3信号通路在该肝保护中的确切作用尚需进一步研究予以证实.
转录因子也是巨噬细胞极化的关键调控分子. 过氧化物酶增殖物激活受体(peroxisome proliferator activated receptors, PPAR)是一类配体依赖性的核转录因子, 存在3种亚型, 即PPARα、PPARβ/δ和PPARγ. 近年来大量研究表明, PPARγ在许多急慢性炎症性疾病中发挥抗炎或促修复效应[41-43]. 而PPARγ发挥抗炎作用的一个重要环节为拮抗LPS等触发的、HMGB1介导的促炎免疫反应[44,45]. 最近研究报道, PPARγ活化在巨噬细胞的极化中发挥着重要作用, 破坏PPARγ可使巨噬细胞的替代活化受阻[41]. 另有研究证明, PPARγ在脂肪组织巨噬细胞M2表型的获得和维持中均发挥着关键作用[46]. 因此, PPARγ被视为巨噬细胞M2极化的主要调节子[41,47]. 而HMGB1作为一种DAMP分子也被报道能够促使巨噬细胞的分化倾向于"M1-样"活化状态[48]. 这就使得利用HMGB1/PPARγ来调控巨噬细胞的M1/M2活化以获得想要的表型和功能成为可能. 在下一步研究工作中, 我们将继续探讨ACLF发病过程中巨噬细胞M1/M2活化的动态变化, 以及利用HMGB1/PPARγ调控巨噬细胞的M1/M2活化对ACLF转归的影响.
近年来, 通过表观遗传调控巨噬细胞极化来改造巨噬细胞已成为学者们探索抗感染与炎症治疗策略的新的研究方向. 表观遗传是指DNA序列不存在改变, 而基因表达发生可遗传的改变, 如DNA甲基化、组蛋白修饰、非编码RNA调控等. 这些表观修饰方式单独或联合作用以影响基因表达与维持[49]. DNA甲基化是最为常见的表观遗传修饰方式, 它在基因表达沉默过程扮演重要角色. 近年研究显示, DNA甲基化与巨噬细胞的M1/M2活化密切相关[50]. 例如, 在四氯化碳诱导的肝纤维化中, PSTPIP2的甲基化促进了巨噬细胞M2活化标记物的表达, 而M1活化标记物的表达则被抑制[51]. 组蛋白的甲基化、乙酰化及磷酸化等修饰方式可组合而成"组蛋白密码", 通过改变染色质紧密结合或疏松状态导致转录抑制或活化, 从而改变细胞的表型及功能状态. 例如, 组蛋白去乙酰化酶(HDAC)是炎症和免疫的重要调节子, 其中HDAC3促进了巨噬细胞的M1活化, 而对于M2巨噬细胞极化来说, 则发挥着"闸"的作用[52]. 另外, H3K27去甲基化酶Jmjd3与巨噬细胞的M2活化密切相关[53]. 虽然非编码RNA对于巨噬细胞极化的调控机制大部分尚未阐明, 到目前为止人们已发现不少非编码RNA, 尤其是miRNA参与巨噬细胞极化调控[54]. 目前已报道的促进M1型巨噬细胞极化的miRNA有miR-155[55]和microRNA-216a[56], 相应的促进M2型的miRNAs有let-7c[57]、miR-125a-5p[58]、miR-223[59]、miR-124[60]等. 表观遗传标记具有长期性, 这已成为靶向巨噬细胞治疗慢性炎症的重要支点. 未来全基因组测序技术的应用及巨噬细胞分离纯度的保证将大大推动巨噬细胞极化的表观遗传调控研究. 目前, 还有许多表观修饰后的基因与巨噬细胞极化的关系仍不明确, 尚需进一步探索.
鉴于巨噬细胞活化的可塑性及功能多样性, 单纯靶向巨噬细胞或者抑制其募集, 很可能会适得其反. 而利用关键介质来促进巨噬细胞功能的早期转换, 以获得促缓解表型(M2), 则有助于减轻肝损伤, 促进肝修复/再生反应以及重要机体功能的恢复.
M1/M2巨噬细胞活化的调控, 已逐渐成为控制急慢性肝脏疾病发生发展及影响疾病转归的重要策略[61]. 加强该领域的研究, 有望为阐明ACLF的发病机制提供新的思路和方向, 也为ACLF的治疗提供新的手段和策略.
近年来, 慢性肝病(肝纤维化)基础上损伤抵抗这一主题逐渐获得临床医生和研究者的关注. 巨噬细胞M1/M2活化及其表型调控在ACLF中的关键作用也越来越被认可. 通过调控巨噬细胞的M1/M2活化以获得促缓解表型, 将成为ACLF治疗的重要新策略.
学科分类: 胃肠病学和肝病学
手稿来源地: 北京市
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): 0
D级 (一般): D
E级 (差): 0
编辑: 崔丽君 电编:张砚梁
| 1. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [PubMed] [DOI] |
| 2. | Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: A curable disease by 2024? J Hepatol. 2015;62:S112-S120. [PubMed] [DOI] |
| 3. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [PubMed] [DOI] |
| 4. | Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576-1587. [PubMed] [DOI] |
| 5. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [PubMed] [DOI] |
| 6. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437; 1437.e1-1437.e9. [PubMed] [DOI] |
| 7. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [PubMed] [DOI] |
| 8. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [PubMed] [DOI] |
| 9. | Bai L, Kong M, Zheng Q, Zhang X, Liu X, Zu K, Chen Y, Zheng S, Li J, Ren F, Lou J, Liu S, Duan Z. Inhibition of the translocation and extracellular release of high-mobility group box 1 alleviates liver damage in fibrotic mice in response to D-galactosamine/lipopolysaccharide challenge. Mol Med Rep. 2016;13:3835-3841. [PubMed] [DOI] |
| 10. | Bai L, Zu K, Zhang X, Ren F, Zheng S, Chen Y, Duan Z. Protective effects and possible mechanisms of hepatic fibrosis against APAP-induced lethal injury. Zhonghua Gan Zang Bing Za Zhi. 2015;23:161-165. [PubMed] [DOI] |
| 11. | Bai L, Liu X, Zheng Q, Kong M, Zhang X, Hu R, Lou J, Ren F, Chen Y, Zheng S, Liu S, Han YP, Duan Z, Pandol SJ. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci Rep. 2017;7:10518. [PubMed] [DOI] |
| 12. | Osawa Y, Hannun YA, Proia RL, Brenner DA. Roles of AKT and sphingosine kinase in the antiapoptotic effects of bile duct ligation in mouse liver. Hepatology. 2005;42:1320-1328. [PubMed] [DOI] |
| 13. | Osawa Y, Seki E, Adachi M, Suetsugu A, Ito H, Moriwaki H, Seishima M, Nagaki M. Role of acid sphingomyelinase of Kupffer cells in cholestatic liver injury in mice. Hepatology. 2010;51:237-245. [PubMed] [DOI] |
| 14. | Bourbonnais E, Raymond VA, Ethier C, Nguyen BN, El-Leil MS, Meloche S, Bilodeau M. Liver fibrosis protects mice from acute hepatocellular injury. Gastroenterology. 2012;142:130-139.e4. [PubMed] [DOI] |
| 15. | Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287-1292. [PubMed] |
| 16. | Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87-93. [PubMed] [DOI] |
| 17. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [PubMed] [DOI] |
| 18. | Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300-1312. [PubMed] [DOI] |
| 19. | Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316-327. [PubMed] [DOI] |
| 20. | Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu Rev Physiol. 2017;79:567-592. [PubMed] [DOI] |
| 21. | Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034-2042. [PubMed] [DOI] |
| 22. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [PubMed] [DOI] |
| 23. | Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832-844. [PubMed] [DOI] |
| 24. | Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage Phenotype in Liver Injury and Repair. Scand J Immunol. 2017;85:166-174. [PubMed] [DOI] |
| 25. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [PubMed] [DOI] |
| 26. | Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307-314. [PubMed] [DOI] |
| 27. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [PubMed] [DOI] |
| 28. | Wan J, Benkdane M, Alons E, Lotersztajn S, Pavoine C. M2 kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am J Pathol. 2014;184:1763-1772. [PubMed] [DOI] |
| 29. | Bai L, Fu L, Li L, Ren F, Zheng Q, Liu S, Han Y, Zheng S, Chen Y, Duan Z. Cellular Mechanisms of Hepatoprotection Mediated by M2-Like Macrophages. Med Sci Monit. 2018;24:2675-2682. [PubMed] [DOI] |
| 30. | Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-342. [PubMed] [DOI] |
| 31. | Tang D, Kang R, Van Houten B, Zeh HJ, Billiar TR, Lotze MT. High mobility group box 1 (HMGB1) phenotypic role revealed with stress. Mol Med. 2014;20:359-362. [PubMed] [DOI] |
| 32. | Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777-787. [PubMed] [DOI] |
| 33. | Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070-1079. [PubMed] [DOI] |
| 34. | Bai L, Jin W, Kong M, Zhang X, Zheng S, Chen Y, Li L, Liu H, Zhu L, Ren F, Li J, Han Y, Duan Z. Injury Resistance in the Setting of Liver Fibrosis Is Accompanied by the Inhibition of High-Mobility Group Box-1 Translocation and Release. Dig Dis. 2018;36:167-176. [PubMed] [DOI] |
| 35. | Zheng QF, Bai L, Duan ZP, Han YP, Zheng SJ, Chen Y, Li JS. M2-like Kupffer cells in fibrotic liver may protect against acute insult. World J Gastroenterol. 2017;23:3655-3663. [PubMed] [DOI] |
| 36. | Possamai LA, Thursz MR, Wendon JA, Antoniades CG. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439-445. [PubMed] [DOI] |
| 37. | Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216-223. [PubMed] [DOI] |
| 38. | Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [PubMed] [DOI] |
| 39. | Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. [PubMed] [DOI] |
| 40. | Kwon YC, Meyer K, Peng G, Chatterjee S, Hoft DF, Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology. 2018; Epub ahead of print. [PubMed] [DOI] |
| 41. | Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. [PubMed] [DOI] |
| 42. | Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62:1551-1562. [PubMed] [DOI] |
| 43. | Han Q, Yuan Q, Meng X, Huo J, Bao Y, Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget. 2017;8:42001-42006. [PubMed] [DOI] |
| 44. | Yuan Z, Luo G, Li X, Chen J, Wu J, Peng Y. PPARγ inhibits HMGB1 expression through upregulation of miR-142-3p in vitro and in vivo. Cell Signal. 2016;28:158-164. [PubMed] [DOI] |
| 45. | Hwang JS, Lee WJ, Kang ES, Ham SA, Yoo T, Paek KS, Lim DS, Do JT, Seo HG. Ligand-activated peroxisome proliferator-activated receptor-δ and -γ inhibit lipopolysaccharide-primed release of high mobility group box 1 through upregulation of SIRT1. Cell Death Dis. 2014;5:e1432. [PubMed] [DOI] |
| 46. | Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613864. [PubMed] [DOI] |
| 47. | Penas F, Mirkin GA, Vera M, Cevey Á, González CD, Gómez MI, Sales ME, Goren NB. Treatment in vitro with PPARα and PPARγ ligands drives M1-to-M2 polarization of macrophages from T. cruzi-infected mice. Biochim Biophys Acta. 2015;1852:893-904. [PubMed] [DOI] |
| 48. | Su Z, Zhang P, Yu Y, Lu H, Liu Y, Ni P, Su X, Wang D, Liu Y, Wang J, Shen H, Xu W, Xu H. HMGB1 Facilitated Macrophage Reprogramming towards a Proinflammatory M1-like Phenotype in Experimental Autoimmune Myocarditis Development. Sci Rep. 2016;6:21884. [PubMed] [DOI] |
| 49. | Hoeksema MA, de Winther MP. Epigenetic Regulation of Monocyte and Macrophage Function. Antioxid Redox Signal. 2016;25:758-774. [PubMed] [DOI] |
| 50. | Zhou D, Yang K, Chen L, Zhang W, Xu Z, Zuo J, Jiang H, Luan J. Promising landscape for regulating macrophage polarization: epigenetic viewpoint. Oncotarget. 2017;8:57693-57706. [PubMed] [DOI] |
| 51. | Yang Y, Wu XQ, Li WX, Huang HM, Li HD, Pan XY, Li XF, Huang C, Meng XM, Zhang L, Lv XW, Wang H, Li J. PSTPIP2 connects DNA methylation to macrophage polarization in CCL4-induced mouse model of hepatic fibrosis. Oncogene. 2018; Epub ahead of print. [PubMed] [DOI] |
| 52. | Chen X, Barozzi I, Termanini A, Prosperini E, Recchiuti A, Dalli J, Mietton F, Matteoli G, Hiebert S, Natoli G. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci USA. 2012;109:E2865-E2874. [PubMed] [DOI] |
| 53. | Hsu AT, Lupancu TJ, Lee MC, Fleetwood AJ, Cook AD, Hamilton JA, Achuthan A. Epigenetic and transcriptional regulation of IL4-induced CCL17 production in human monocytes and murine macrophages. J Biol Chem. 2018;293:11415-11423. [PubMed] [DOI] |
| 54. | Li H, Jiang T, Li MQ, Zheng XL, Zhao GJ. Transcriptional Regulation of Macrophages Polarization by MicroRNAs. Front Immunol. 2018;9:1175. [PubMed] [DOI] |
| 55. | Bala S, Csak T, Saha B, Zatsiorsky J, Kodys K, Catalano D, Satishchandran A, Szabo G. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64:1378-1387. [PubMed] [DOI] |
| 56. | Yang S, Li J, Chen Y, Zhang S, Feng C, Hou Z, Cai J, Wang Y, Hui R, Lv B, Zhang W. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochim Biophys Acta Mol Basis Dis. 2018; Epub ahead of print. [PubMed] |
| 57. | Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542-6549. [PubMed] [DOI] |
| 58. | Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, Thannickal VJ, Abraham E, Liu G. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428-35436. [PubMed] [DOI] |
| 59. | Yuan X, Berg N, Lee JW, Le TT, Neudecker V, Jing N, Eltzschig H. MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol. 2018;104:515-524. [PubMed] [DOI] |
| 60. | Sun Y, Qin Z, Li Q, Wan JJ, Cheng MH, Wang PY, Su DF, Yu JG, Liu X. MicroRNA-124 negatively regulates LPS-induced TNF-α production in mouse macrophages by decreasing protein stability. Acta Pharmacol Sin. 2016;37:889-897. [PubMed] [DOI] |
| 61. | Vannella KM, Wynn TA. Mechanisms of Organ Injury and Repair by Macrophages. Annu Rev Physiol. 2017;79:593-617. [PubMed] [DOI] |