修回日期: 2019-12-05
接受日期: 2019-12-13
在线出版日期: 2019-12-28
肝硬化是各种慢性肝病的终末阶段, 常伴有门静脉高压. 自发性脾肾分流(spontaneous splenorenal shunt, SSRS)指的是从脾静脉到肾静脉之间丰富并增粗的异常血管, 其形成机制可能与先天存在的小血管扩张有关, 也可能与新生血管有关. SSRS可有效降低门静脉压力, 但也会减少入肝血流, 其已被证实是导致肝移植术后肝脏血流灌注不足的重要原因之一; 此外, SSRS导致血液中的有毒物质不能经肝脏充分代谢而直接进入体循环, 进而增加肝性脑病的发生风险. 目前, 介入和外科手术是SSRS的主要治疗方法.
核心提要: 自发性脾肾分流可降低门静脉压力, 但也可导致肝性脑病.
引文著录: 易芳芳, 白朝辉, 许向波, 祁兴顺. 肝硬化患者自发性脾肾分流的研究进展. 世界华人消化杂志 2019; 27(24): 1502-1508
Revised: December 5, 2019
Accepted: December 13, 2019
Published online: December 28, 2019
Liver cirrhosis is the end stage of various chronic liver diseases. Spontaneous splenorenal shunt (SSRS) refers to abnormal blood vessels from the splenic vein to the renal vein, which are rich and thickened. SSRS formation may be due to the dilatation of pre-existing venules or neovascularization. SSRS can effectively reduce portal vein pressure, but it can also lead to a decrease of hepatic perfusion, which may be one of the reasons for insufficient hepatic perfusion after liver transplantation. In addition, toxic substances in the blood cannot be fully metabolized by the liver and directly enter the systemic circulation, leading to the development of hepatic encephalopathy. The treatment methods for SSRS include intervention and operation.
- Citation: Yi FF, Bai ZH, Xu XB, Qi XS. Advances in research of spontaneous splenorenal shunt in patients with liver cirrhosis. Shijie Huaren Xiaohua Zazhi 2019; 27(24): 1502-1508
- URL: https://www.wjgnet.com/1009-3079/full/v27/i24/1502.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v27.i24.1502
肝硬化常表现为门静脉血流阻力逐渐增加, 导致门静脉高压[1]. 肝静脉压力梯度间接代表门静脉压力, 正常值范围是3-5 mmHg; 当肝静脉压力梯度超过10 mmHg, 称为临床显著门静脉高压[2]. 除了食管胃底静脉曲张、腹水和脾大, 自发性门体分流也是门静脉高压的表现之一[3-5], 其主要包括脾肾分流、胃肾分流、脐静脉开通; 其中, 脾肾分流是最常见的类型[6,7].
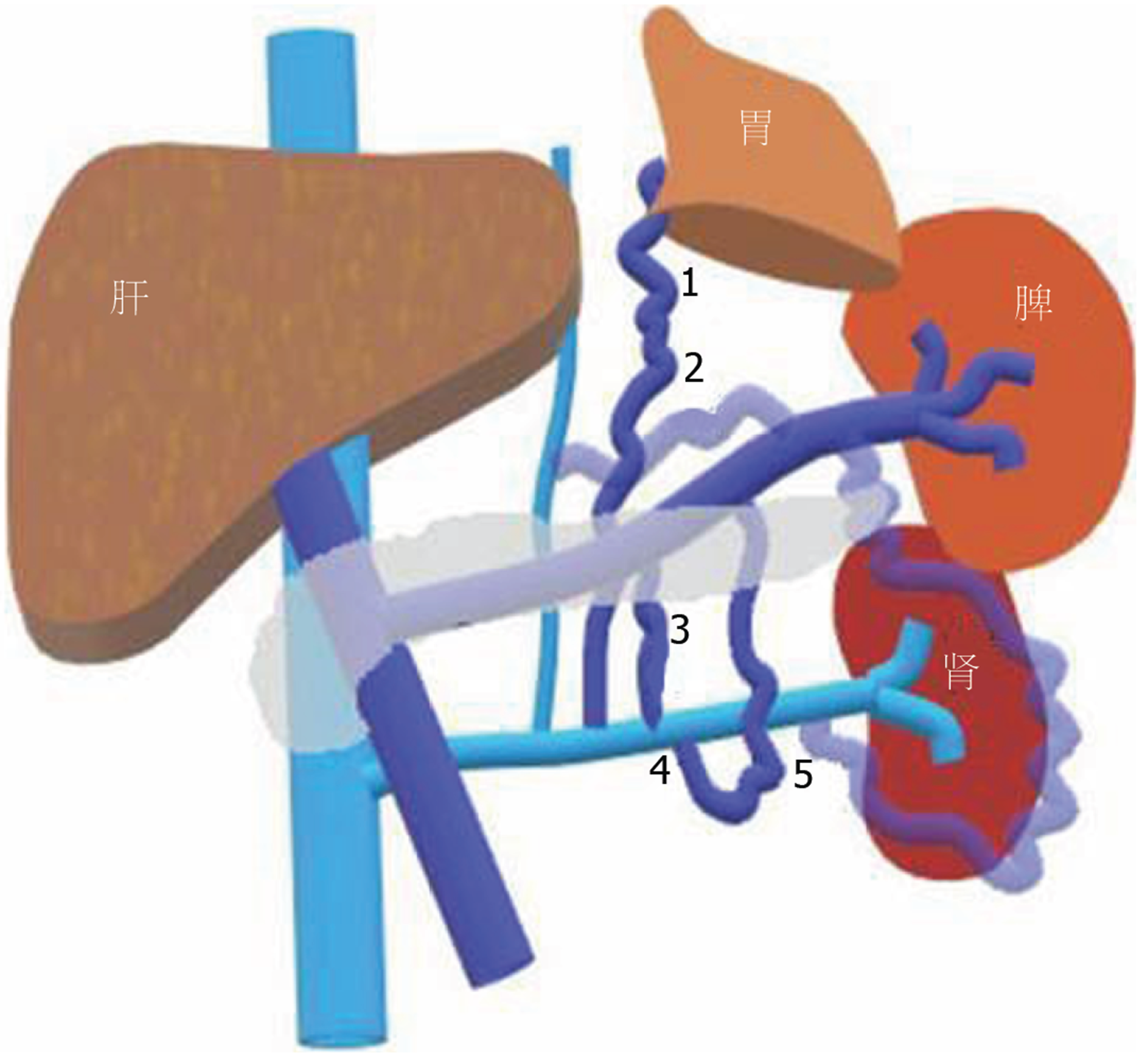
自发性脾肾分流(spontaneous splenorenal shunt, SSRS)指的是从脾静脉到肾静脉之间丰富并增粗的异常血管(图1), SSRS分为两种: 直接SSRS指的是脾静脉和肾静脉之间异常的交通支; 间接SSRS, 也叫胃肾分流, 指的是胃短静脉或胃后静脉与左肾静脉之间异常的交通支[8]. SSRS的影像学检查方法包括彩色多普勒超声、增强CT/MRI及血管造影, SSRS在临床中并不少见, 但未引起足够的重视. 近年来有研究报道, SSRS的发生可能与先天小血管的扩张或新生血管的生成有关[9]. 一项病例对照研究发现, 与无SSRS组相比, SSRS组肝性脑病的发生率显著增高(0% vs 46%)[10], 其原因可能是SSRS患者血液中的有毒物质未经肝脏代谢直接进入体循环而引起肝性脑病[11]. 目前, 临床工作中关于SSRS的治疗方法, 主要包括介入和外科手术, 但目前并无相关指南可以参考. 本文主要回顾肝硬化患者SSRS的患病率、发病机制、影像学特征、临床预后以及治疗策略.
肝硬化患者SSRS的患病率大约是10.5%-21%. 不同研究报道的患病率差异主要与检查方法和患者特征有关. 一项纳入105例肝硬化患者的研究中, 增强CT或MRI检查SSRS的患病率为10.5%[12]. 另一项纳入326例肝硬化患者的研究中, 彩色多普勒超声检查SSRS的患病率为13.8%[13]. 此外, 一项纳入109例肝硬化患者的研究也通过彩色多普勒超声检查SSRS, 患病率为21%[14].
肝门静脉系统包括门静脉、脾静脉、肠系膜静脉、胃左静脉、胃右静脉和脐静脉等[15]. 门静脉无静脉瓣膜, 当门静脉高压时, 血液可以通过门静脉属支逆流建立侧支循环[16]. 从解剖学角度, SSRS分为三类: 垂直连接膈下静脉、连接性腺静脉和直接连接左肾静脉. 然而, 从脾静脉发出的血管又分为内侧、前外侧和后外侧[17]. 内侧沿胰腺后组织横向弯曲, 然后与膈下静脉汇合. 前外侧沿肾旁前间隙向前走行, 侧转后沿降结肠背侧走行, 穿过肾筋膜进入肾周间隙, 继续向内侧走行, 最后与膈下静脉或肾静脉连接; 后外侧沿肾旁前间隙向后行, 并穿过肾周间隙, 然后连接性腺静脉或肾静脉.
有研究者提出[18-20]SSRS的形成是先天存在的小血管扩张. 扩张的小血管指的是胰腺背部的脾静脉与肾静脉之间的小静脉开通[21]. 当门静脉压力梯度>10 mmHg时, 门静脉的部分血液经过脾静脉与肾静脉之间的小静脉, 最后汇入下腔静脉, 形成SSRS. 扩张的小血管也可能是先天存在的胚胎静脉, 随着门静脉压力升高, 连接脾静脉与左肾静脉[10].
近年来, 有研究发现[9], 侧支血管的形成不仅是门静脉及属支的开放, 而且与新生血管有关. 起初, 血管扩张、血管通透性增加, 通过血管平滑肌细胞脱落, 血管壁失稳以及细胞外基质降解, 导致增生的内皮细胞迁移、聚集并形成管状结构; 最后, 内皮细胞募集周细胞, 完成新生血管的过程[22]. 促进血管生成的调节因子包括血管内皮生长因子(vascular endothelial growth factor, VEGF)、转化生长因子、肝细胞生长因子、白细胞介素和血管生成素等; 其中, VEGF是最重要的血管生成因子[16]. 已有动物实验研究证实[23], 门静脉高压老鼠形成的门体侧支循环与VEGF诱导的新生血管有关. 上述结果为门静脉高压的病理生理学研究提供了新的方向. 然而, 动物实验的局限性在于只模拟了人类肝病门静脉高压某些特定的方面, 并不能准确地反映门静脉高压在人类肝病中的真实状态. 因此, 新生血管与SSRS的关系仍需进一步研究.
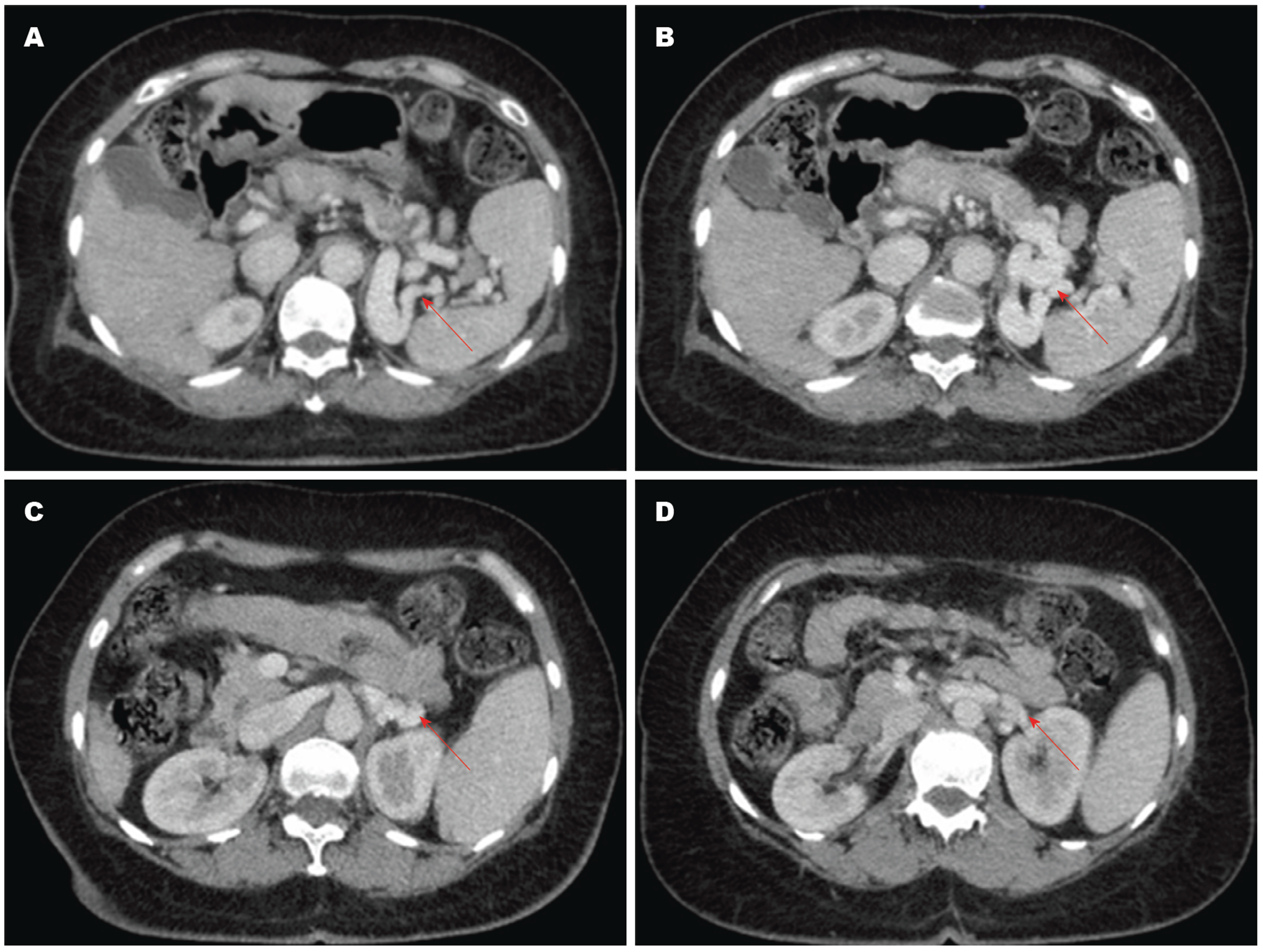
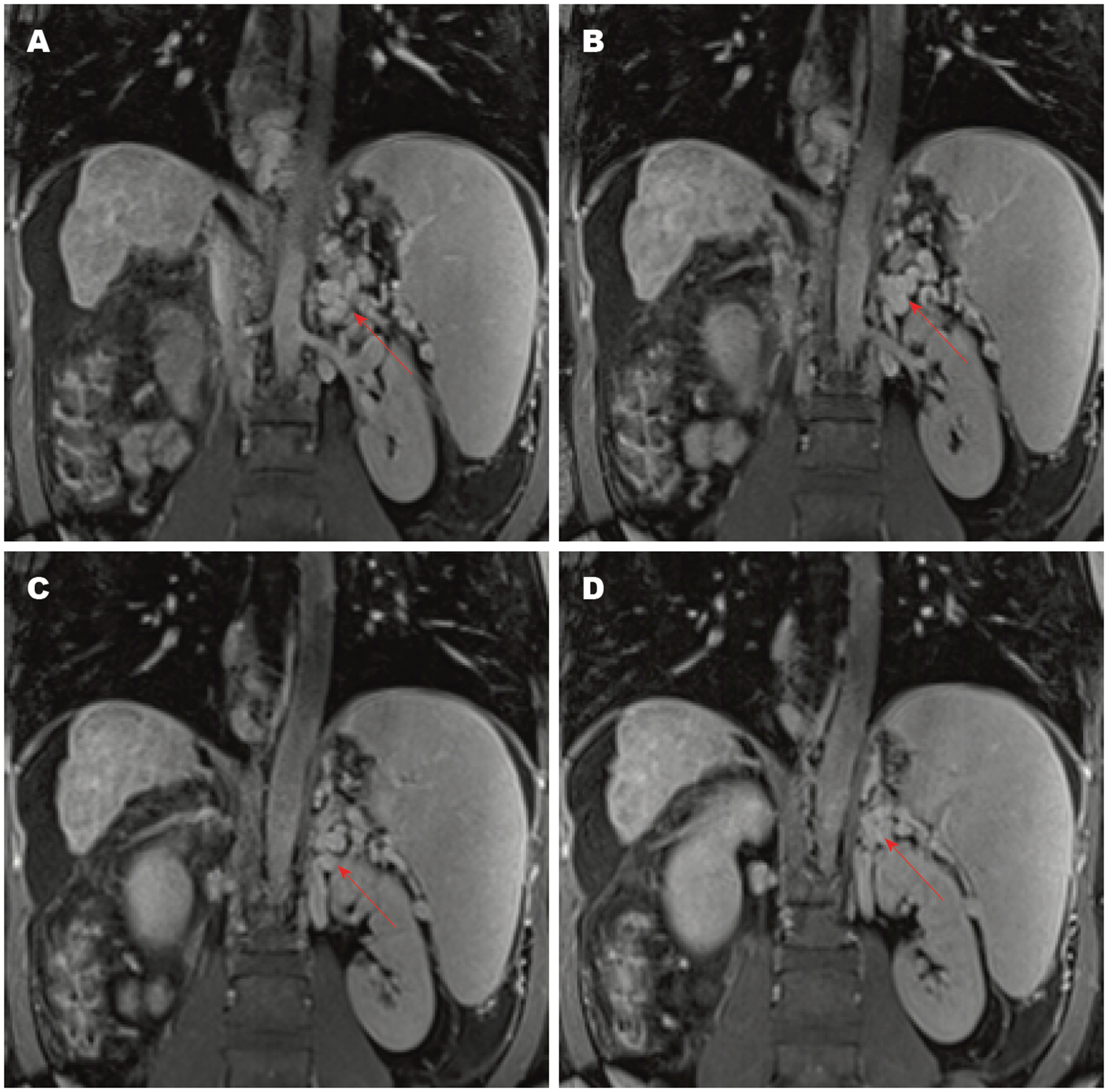
SSRS的影像学诊断方法包括彩色多普勒超声、增强CT/MRI和血管造影[24,25]. 彩色多普勒超声表现为脾静脉和左肾静脉之间的圆形或椭圆形红蓝相间的团块样血管[26], 具有无创性和简便性, 适用于评估门静脉主干和侧支血管的血流方向[27]. 增强CT/MRI能清晰地显示SSRS的图像(图2, 图3), 表现为均匀强化的团块状影, 其密度与脾静脉和肾静脉一致且高于肝、脾及邻近软组织[28]. 多层螺旋CT三维重建技术可更清晰地显影出SSRS的开放和严重程度. 平扫CT也可显示脾脏周围团块样血管, 但不能诊断SSRS; 只有使用造影剂后, 脾静脉和左肾静脉之间的异常血管被强化时, 才可诊断SSRS[29]. 血管造影是诊断SSRS的金标准[30], 但具有侵袭性, 价格昂贵, 不适用于严重肝功能障碍的患者[29,31].
SSRS可将血液从高压的门静脉分流至低压的全身血管, 进而降低门静脉压力, 但也带来了新的临床问题[32]. 一方面, 血流方向与疾病严重程度相关, SSRS大部分是离肝血流, 导致肝脏血流减少、肝脏缺血、肝功能进一步减退[33]. 另一方面, 侧支血管的血流方向是导致肝性脑病的重要因素, 大量的离肝血流未经过肝脏代谢直接进入血液循环, 导致肝性脑病的发病率增加[34]. 有研究提示[11], 46%-70%的难治性肝性脑病患者存在粗大的自发性门体分流, 而自发性门体分流最常见的是SSRS. 因此, SSRS在降低门静脉压力的同时, 增加了肝性脑病的风险.
肝移植患者中SSRS的患病率为20%-35%[35,36]. SSRS在肝移植术前降低门静脉压力, 进而延长终末期肝病患者等待肝移植时间; 然而, SSRS在肝移植术后可能导致部分肝移植患者出现门静脉血流不足的现象, 进而影响移植物的血流灌注[37]. 当SSRS的直径超过10 mm时, 可增加肝移植手术风险[38], 严重者可导致术中凝血功能障碍, 术后门静脉血栓形成, 甚至导致患者死亡. 因此, 门静脉高压的肝移植患者应警惕是否合并SSRS.
球囊阻断逆行经静脉栓塞术(balloon-occluded retrograde transvenous obliteration, BRTO)是经颈静脉或股静脉逆行左肾静脉插管, 随后通过球囊栓塞和缓慢注入硬化剂阻断SSRS的一项介入手术[39,40]. BRTO最早用于治疗肝硬化门脉高压导致的胃静脉曲张破裂出血[1]; 当患者合并SSRS, BRTO与内镜下组织胶/硬化剂治疗胃静脉曲张出血相比, 可以降低异位栓塞的风险[41]. BRTO通过阻断SSRS, 进而增加门静脉血流量, 增强肝脏对神经毒性物质代谢, 从而防治肝性脑病[42]. 2014年, Inoue等[43]纳入了19例因SSRS导致肝性脑病的患者接受BRTO治疗, 术后肝性脑病的缓解率为100%, 随访期间有6例患者发生死亡, 但均与BRTO手术无关(3例肝衰竭、2例肝癌、1例脑梗死).
门体分流栓塞术适用于门体分流导致的难治性肝性脑病患者[11,44]. 栓塞的材料包括线圈、封堵器和弹簧圈[45,46]. 栓塞的途径包括经皮、经肝、经颈静脉和股静脉[46]. 2016年, Lynn等[46]纳入20例患者并回顾性研究了门体分流栓塞术治疗难治性肝性脑病的疗效, 其中SSRS最常见(60%), 栓塞术的材料包括线圈(75%)、封堵器(20%)、线圈与封堵器联合(5%), 途径包括为经肝(25%)、经股静脉(30%)、经颈静脉(25%)、经脐静脉(5%)、经右腋静脉(15%); 结果表明, 门体分流栓塞术后所有患者肝性脑病的症状均缓解, 且术后并发症的发生率为10%, 此外, 门体分流栓塞术还降低了1年内因肝性脑病再入院的风险[46]. 因此, 门体分流栓塞术可能是一种安全有效的治疗门体分流性肝性脑病的方法.
经颈静脉肝内门体分流术(transjugular intrahepatic portosystemic shunt, TIPS)及SSRS都可以降低门静脉压力[47], 减少肝脏血流灌注并增加肝性脑病的风险. 2013年, 何创业等[48]纳入了9例患者行TIPS联合封堵器治疗肝硬化门脉高压伴自发性脾胃肾分流; 5例患者先行TIPS术, 术后4例发生严重的肝性脑病, 后经封堵治疗肝性脑病缓解; 其余4例患者直接行TIPS联合封堵治疗, 术后均无肝性脑病发生. 因此, 推荐介入医生在TIPS的同时联合封堵器栓塞自发性门体分流道, 这既能有效降低门静脉压力, 又能减少肝性脑病的发生风险[48].
脾切除是肝移植患者合并SSRS的根治性手术, 适用于SSRS直径>10 mm、无门静脉血栓、伴有脾动脉瘤、严重脾功能亢进和巨脾的患者[36,49]. 脾切除可有效减少SSRS的血流量, 快速纠正肝硬化患者的血小板减少症. 然而, 肝移植术中同时施行脾切除对手术技术要求高, 手术难度大, 术后早期感染风险高, 可能出现严重的脓毒血症、门静脉血栓、免疫相关并发症等[50].
左肾静脉结扎术(left renal vein ligation, LRVL)适用于门静脉开通且SSRS直径>10 mm的患者[51]. LRVL可通过夹闭和阻断左肾静脉与下腔静脉汇合处, 增加门静脉血流量[51,52]. 与脾切除术相比, LRVL是一种相对安全、简便的阻断脾肾分流且增加向肝血流的手术方法[36,53,54], 但术后可能出现暂时性血清肌酐升高, 甚至持续性肾功能损害[51]. 2015年, Golse等[36]纳入29例接受肝移植的患者, 术前影像学检查SSRS的直径>10 mm; 根据肝移植术中SSRS患者接受的手术方式, 分为脾切除术组(22例)和LRVL组(7例); 结果表明, 两组术后的门静脉血流量均增加, 9例脾切除术组患者和1例LRVL组患者出现了慢性肾功能不全. 随访期间, 两组患者术后并发症的发生率无显著差异, 脾切除术组的5年生存率为100%, 而LRVL组有1例患者在随访的第11个月死于肠缺血, 与LRVL手术无关.
肾静脉-门静脉吻合术(renoportal anastomosis, RPA)适用于SSRS直径>10 mm、门静脉完全血栓、门静脉直径狭窄的患者[51,55]. 2005年, Marubashi等[56]纳入3例接受肝移植手术前合并SSRS及门静脉血栓的患者, RPA术后所有患者吻合口均开通, 2例患者术后出现肾功能损害, 但当肾脏灌注恢复正常后, 肾功能恢复正常. 因此, 肝移植联合RPA手术也可能是终末期肝病患者合并SSRS及门静脉血栓的治疗方式.
肝硬化合并SSRS的患病率高. 传统观点认为SSRS是先天小静脉的开放, 最近的研究观点则表明SSRS与新生血管有关. SSRS可降低门静脉压力, 但增加了肝性脑病的风险及肝移植手术并发症的风险. 因此, 临床实践中应依据SSRS患者的具体病情选择个体化的治疗方案.
学科分类: 胃肠病学和肝病学
手稿来源地: 辽宁省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): 0
D级 (一般): D, D
E级 (差): 0
编辑: 王禹乔 电编:刘继红
| 1. | 王 芳, 刘 仕倩, 曾 西, 吴 楠楠, 张 静, 陈 明锴. 食管胃底静脉曲张伴自发性分流血管栓塞治疗的研究进展. 胃肠病学和肝病学杂志. 2018;27:346-350,355. [DOI] |
| 3. | Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424. [PubMed] [DOI] |
| 4. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [PubMed] [DOI] |
| 5. | Dilawari JB, Chawla YK. Spontaneous (natural) splenoadrenorenal shunts in extrahepatic portal venous obstruction: a series of 20 cases. Gut. 1987;28:1198-1200. [PubMed] [DOI] |
| 6. | Bandali MF, Mirakhur A, Lee EW, Ferris MC, Sadler DJ, Gray RR, Wong JK. Portal hypertension: Imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol. 2017;23:1735-1746. [PubMed] [DOI] |
| 7. | Wu Q, Shen L, Chu J, Ma X, Jin B, Meng F, Chen J, Wang Y, Wu L, Han J, Zhang W, Ma W, Wang H, Li H. Characterization of uncommon portosystemic collateral circulations in patients with hepatic cirrhosis. Oncol Lett. 2015;9:347-350. [PubMed] [DOI] |
| 9. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [PubMed] [DOI] |
| 10. | Ohnishi K, Sato S, Saito M, Terabayashi H, Nakayama T, Saito M, Chin N, Iida S, Nomura F, Okuda K. Clinical and portal hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol. 1986;81:450-455. [PubMed] |
| 11. | Franzoni Lde C, de Carvalho FC, Garzon RG, Yamashiro Fda S, Augusti L, Santos LA, Dorna Mde S, Baima JP, Lima TB, Caramori CA, Silva GF, Romeiro FG. Embolization of splenorenal shunt associated to portal vein thrombosis and hepatic encephalopathy. World J Gastroenterol. 2014;20:15910-15915. [PubMed] [DOI] |
| 12. | Qi X, Qi X, Zhang Y, Shao X, Wu C, Wang Y, Wang R, Zhang X, Deng H, Hou F, Li J, Guo X. Prevalence and Clinical Characteristics of Spontaneous Splenorenal Shunt in Liver Cirrhosis: A Retrospective Observational Study Based on Contrast-Enhanced Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) Scans. Med Sci Monit. 2017;23:2527-2534. [PubMed] [DOI] |
| 13. | Zardi EM, Uwechie V, Caccavo D, Pellegrino NM, Cacciapaglia F, Di Matteo F, Dobrina A, Laghi V, Afeltra A. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44:76-83. [PubMed] [DOI] |
| 14. | von Herbay A, Frieling T, Häussinger D. Color Doppler sonographic evaluation of spontaneous portosystemic shunts and inversion of portal venous flow in patients with cirrhosis. J Clin Ultrasound. 2000;28:332-339. [PubMed] [DOI] |
| 15. | Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr. 2018;177:285-294. [PubMed] [DOI] |
| 17. | Achiwa S, Hirota S, Kako Y, Takaki H, Kobayashi K, Yamakado K. Radiological anatomy of spontaneous splenorenal shunts in patients with chronic liver disease. Jpn J Radiol. 2017;35:206-214. [PubMed] [DOI] |
| 19. | Harmanci O, Bayraktar Y. Clinical characteristics of idiopathic portal hypertension. World J Gastroenterol. 2007;13:1906-1911. [PubMed] [DOI] |
| 20. | Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141-155. [PubMed] [DOI] |
| 21. | Wind P, Alves A, Chevallier JM, Gillot C, Sales JP, Sauvanet A, Cuénod CA, Vilgrain V, Cugnenc PH, Delmas V. Anatomy of spontaneous splenorenal and gastrorenal venous anastomoses. Review of the literature. Surg Radiol Anat. 1998;20:129-134. [PubMed] |
| 22. | Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149-161. [PubMed] |
| 23. | Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886-894. [PubMed] [DOI] |
| 24. | 张 灿环, 刘 鸿雁, 张 炳, 高 健, 王 国华, 方 明, 王 钦习. 多层螺旋CT门静脉成像评价脾肾静脉分流的价值. 中国临床医学影像杂志. 2018;29:277-280. [DOI] |
| 25. | 张 放, 杨 岳松, 张 丕利, 张 益军, 彭 志海. 磁共振、彩色多普勒及间接门脉数字减影血管造影在门静脉系统显像中的对照研究. 临床外科杂志. 2001;9:161-163. [DOI] |
| 28. | 陈 卫霞, 周 翔平, 闵 鹏秋, 宋 彬, 黄 娟, 许 崇永, 易 凤琼, 杨 敏. 门静脉高压脾静脉与左肾静脉自发交通CT表现. 临床放射学杂志. 1999;18:284-285. [DOI] |
| 29. | Takayasu K, Moriyama N, Shima Y, Yamada T, Kobayashi C, Musha H, Okuda K. Sonographic detection of large spontaneous spleno-renal shunts and its clinical significance. Br J Radiol. 1984;57:565-570. [PubMed] [DOI] |
| 30. | Farid N, Balkanci F, Guran S, Senaati S, Besim A. A digital splenoportography: more sensitive method of detecting spontaneous splenorenal shunt. Angiology. 1991;42:754-759. [PubMed] [DOI] |
| 31. | Qi X, Ye C, Hou Y, Guo X. A large spontaneous intrahepatic portosystemic shunt in a cirrhotic patient. Intractable Rare Dis Res. 2016;5:58-60. [PubMed] [DOI] |
| 35. | Chikamori F, Nishida S, Selvaggi G, Tryphonopoulos P, Moon JI, Levi DM, Kato T, Island ER, Maki A, Tekin A, Tzakis AG. Effect of liver transplantation on spleen size, collateral veins, and platelet counts. World J Surg. 2010;34:320-326. [PubMed] [DOI] |
| 36. | Golse N, Mohkam K, Rode A, Mezoughi S, Demian H, Ducerf C, Mabrut JY. Surgical Management of Large Spontaneous Portosystemic Splenorenal Shunts During Liver Transplantation: Splenectomy or Left Renal Vein Ligation? Transplant Proc. 2015;47:1866-1876. [PubMed] [DOI] |
| 38. | Kim H, Yoon KC, Lee KW, Yi NJ, Lee HW, Choi Y, Oh D, Kim HS, Hong SK, Ahn SW, Suh KS. Tips and pitfalls in direct ligation of large spontaneous splenorenal shunt during liver transplantation. Liver Transpl. 2017;23:899-906. [PubMed] [DOI] |
| 39. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [PubMed] [DOI] |
| 40. | Nakai M, Sato M, Sahara S, Kawai N, Kimura M, Maeda Y, Ibata Y, Higashi K. Transhepatic catheter-directed thrombolysis for portal vein thrombosis after partial splenic embolization in combination with balloon-occluded retrograde transvenous obliteration of splenorenal shunt. World J Gastroenterol. 2006;12:5071-5074. [PubMed] [DOI] |
| 42. | Numata K, Tanaka K, Kiba T, Saito S, Shirato K, Kitamura T, Sekihara H. Use of balloon-occluded retrograde transvenous obliteration with ethanolamine oleate for the treatment of hepatic encephalopathy in a cirrhotic patient with a large spontaneous splenorenal shunt. J Gastroenterol. 1998;33:424-427. [PubMed] [DOI] |
| 43. | Inoue H, Emori K, Toyonaga A, Oho K, Kumamoto M, Haruta T, Mitsuyama K, Tsuruta O, Sata M. Long term results of balloon-occluded retrograde transvenous obliteration for portosystemic shunt encephalopathy in patients with liver cirrhosis and portal hypertension. Kurume Med J. 2014;61:1-8. [PubMed] [DOI] |
| 44. | Kessler J, Trerotola SO. Use of the Amplatzer Vascular Plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Interv Radiol. 2006;17:135-140. [PubMed] [DOI] |
| 45. | Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, Brookes J, Thalassinos E, Burroughs AK, Cordoba J, Nevens F; EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448-2457. [PubMed] [DOI] |
| 46. | Lynn AM, Singh S, Congly SE, Khemani D, Johnson DH, Wiesner RH, Kamath PS, Andrews JC, Leise MD. Embolization of portosystemic shunts for treatment of medically refractory hepatic encephalopathy. Liver Transpl. 2016;22:723-731. [PubMed] [DOI] |
| 48. | 何 创业, 祁 兴顺, 帖 君, 柏 明, 白 苇, 郭 文刚, 牛 静, 殷 占新, 韩 国宏. 经颈内静脉肝内门体分流术联合封堵器封堵治疗肝硬化门脉高压伴自发性脾、胃-肾分流. 介入放射学杂志. 2013;22:801-805. [DOI] |
| 49. | Yu H, Guo S, Wang L, Dong Y, Tian G, Mu S, Zhang H, Li D, Zhao S. Laparoscopic Splenectomy and Esophagogastric Devascularization for Liver Cirrhosis and Portal Hypertension Is a Safe, Effective, and Minimally Invasive Operation. J Laparoendosc Adv Surg Tech A. 2016;26:524-530. [PubMed] [DOI] |
| 50. | Cescon M, Sugawara Y, Kaneko J, Ohtsuka H, Takayama T, Makuuchi M. Restoration of portal vein flow by splenorenal shunt ligation and splenectomy after living-related liver transplantation. Hepatogastroenterology. 2001;48:1453-1454. [PubMed] |
| 51. | Tang R, Han D, Li M, Shen S, Huang X, Zhao W, Dong J. Left renal vein ligation for large splenorenal shunt during liver transplantation. ANZ J Surg. 2017;87:767-772. [PubMed] [DOI] |
| 52. | Nguyen MC, Sage Silski L, Alebrahim M, Black S, Elkhammas E, Washburn K, El-Hinnawi A. Left Renal Vein Ligation for Spontaneous Splenorenal Shunts During Deceased-Donor Orthotopic Liver Transplant Is Safe and Can Mitigate Complications from Portal Steal: A Case Series. Exp Clin Transplant. 2018;. [PubMed] [DOI] |
| 53. | Slater RR, Jabbour N, Abbass AA, Patil V, Hundley J, Kazimi M, Kim D, Yoshida A, Abouljoud M. Left renal vein ligation: a technique to mitigate low portal flow from splenic vein siphon during liver transplantation. Am J Transplant. 2011;11:1743-1747. [PubMed] [DOI] |
| 54. | Castillo-Suescun F, Oniscu GC, Hidalgo E. Hemodynamic consequences of spontaneous splenorenal shunts in deceased donor liver transplantation. Liver Transpl. 2011;17:891-895. [PubMed] [DOI] |
| 55. | Miyamoto A, Kato T, Dono K, Umeshita K, Kawabata R, Hayashi S, Kubota M, Kobayashi S, Nagano H, Nakamori S, Sakon M, Monden M. Living-related liver transplantation with renoportal anastomosis for a patient with large spontaneous splenorenal collateral. Transplantation. 2003;75:1596-1598. [PubMed] [DOI] |
| 56. | Marubashi S, Dono K, Nagano H, Gotoh K, Takahashi H, Hashimoto K, Miyamoto A, Takeda Y, Umeshita K, Kato T, Monden M. Living-donor liver transplantation with renoportal anastomosis for patients with large spontaneous splenorenal shunts. Transplantation. 2005;80:1671-1675. [PubMed] [DOI] |