修回日期: 2018-05-27
接受日期: 2018-06-02
在线出版日期: 2018-07-08
观察4种纤维蛋白相关标志物(fibrin-related markers, FRMs): 纤维蛋白单体(fibrin monomer, FM)、D-二聚体(D-Dimer, D-D)、纤维蛋白原(fibrinogen, FIB)和(或)纤维蛋白降解产物(fibrin degradation products, FDP)和FIB在不同严重程度急性胰腺炎(acute pancreatitis, AP)中的动态变化, 评价其对AP伴持续器官功能衰竭(persistent organ failure, POF)和胰腺坏死(pancreatic necrosis, PN)的预测价值.
我们的研究前瞻性的纳入152例AP病人. AP的严重性根据最终出现的POF和PN判定, 检测入院后第1、2、3和7天AP病人血浆中4种FRMs的浓度, 比较4种FRMs在不同严重程度AP中的变化, 采用ROC曲线计算FM、D-D、FDP预测POF和PN的临界值及其敏感性, 特异性, 阳性预测值和阴性预测值, 并与常用的C-反应蛋白(C-reaction protein, CRP)和乳酸脱氢酶(lactate dehydrogenase, LDH)作比较.
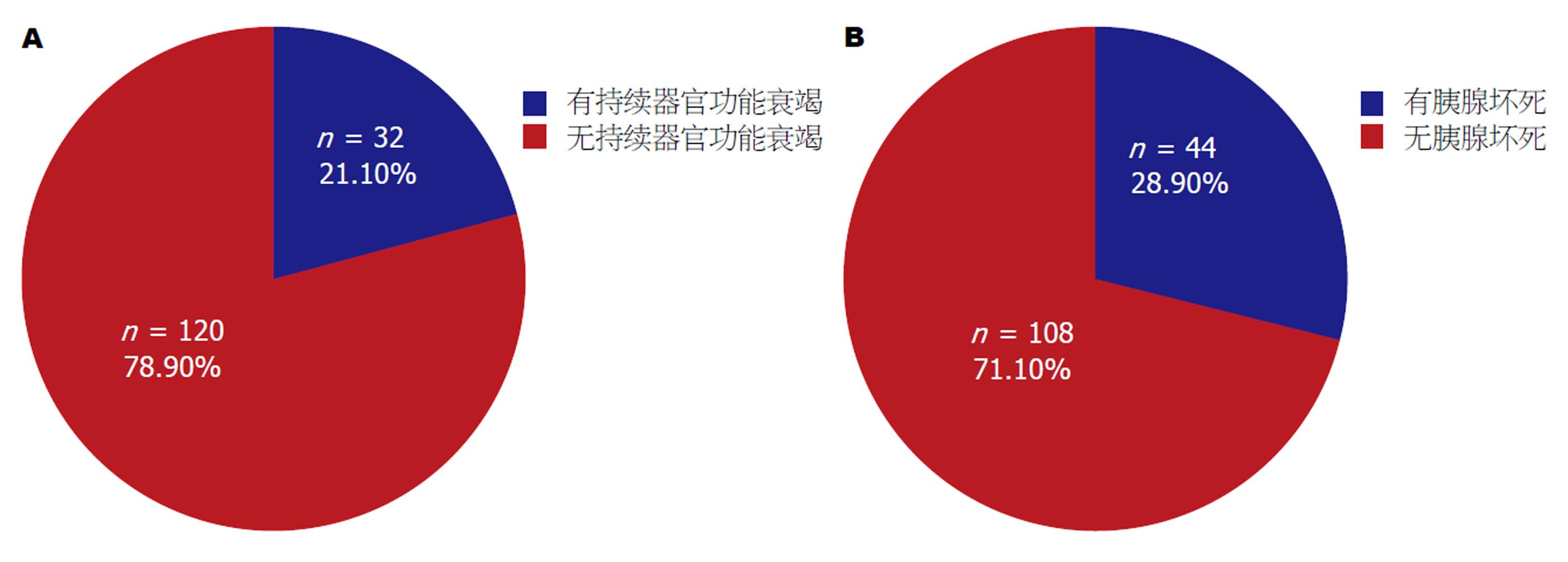
152例AP病人中, 32例发生POF, 44例发生PN. 血清FM在有无POF的AP病人组间差异无显著性; PN组血清FM在入院后第1(P = 0.043)、2(P = 0.008)、3(P = 0.007)和7天(P = 0.002)明显高于无PN组. 入院后第1(P = 0.001)、2(P = 0.004)、3(P = 0.000)和7天(P = 0.002)POF组病人的D-D高于无POF组; 入院后第1(P = 0.023)、2 (P = 0.045)、3(P = 0.000)和7天(P = 0.000), PN组病人的D-D高于无PN组. 入院后1(P = 0.000)、2(P = 0.000)、3(P = 0.000)和7天(P = 0.000), POF组的FDP高于无POF组; 入院后第2(P = 0.021)、3(P = 0.000)和7天(P = 0.000), PN组的FDP高于无PN组. 入院后1 wk在有无POF组和有无PN组间血浆FIB差异无显著性; ROC曲线分析表明, 入院后第1天FDP (AUC = 0.711)和D-D (AUC = 0.693)对POF的预测优于常用的CRP(AUC = 0.615)和LDH(AUC = 0.672). 入院后第3天D-D(AUC = 0.832)和FDP(AUC = 0.814)对PN有较好的预测价值, 优于LDH(AUC = 0.639)和CRP(AUC = 0.706).
入院后1 wk 4种FRMs在AP伴POF和PN组中高于AP无POF和PN组, D-D和FDP与AP的严重性明显相关, 对AP的严重程度有一定的预测价值, 可以作为AP发生POF和AP发生PN的较好的辅助诊断指标.
核心提要: 本研究检测了入院后第1、2、3和7天的血清4种纤维蛋白相关标志物(fibrin-related markers, FRMs)在不同严重程度急性胰腺炎(acute pancreatitis, AP)中的变化; 通过ROC曲线, 分析了FRMs对AP发生持续器官功能衰竭和胰腺坏死的预测价值, 并与常用的预测AP严重性的指标: 如C-反应蛋白和乳酸脱氢酶等做了比较.
引文著录: 雷静静, 周力, 熊灿, 刘琦, 邓宛航. 急性胰腺炎血浆纤维蛋白相关标志物的动态变化及临床意义. 世界华人消化杂志 2018; 26(19): 1176-1185
Revised: May 27, 2018
Accepted: June 2, 2018
Published online: July 8, 2018
To investigate whether the four fibrin-related markers (FRMs) fibrin monomer (FM), D-dimer (D-D), fibrinogen (FIB), and fibrin degradation products (FDP) reflect the extent of coagulation activation in vivo and to assess the predictive value of the FRMs in determining persistent organ failure (POF) and pancreatic necrosis (PN) in acute pancreatitis (AP) patients.
One hundred and fifty-two AP patients were included in this prospective observational study. The final outcome was disease severity assessed by presence of POF and PN. The levels of the four FRMs were measured on days 1, 2, 3, and 7 after admission. ROC curves were used to compare the sensitivity, specificity, PPV, and NPV of FM, D-D, and FDP in predicting POF and PN with those of regular biochemical markers C-reaction protein (CRP) and lactate dehydrogenase (LDH).
Of the 152 patients included, 32 had POF and 44 had PN. There was no significant difference in serum FM levels between AP with POF and AP without POF at the first week after admission. Patients with PN had significantly higher FM than those without PN on day 1 (P = 0.043), day 2 (P = 0.008), day 3 (P = 0.001), and day 7 (P = 0.002) after admission. D-D was significantly higher in patients with POF than in those without on day 1 (P = 0.001), day 2 (P = 0.004), day 3 (P = 0.000), and day 7 (P = 0.002). Patients with PN had significantly higher D-D on day 1 (P = 0.023), day 2 (P = 0.045), day 3 (P = 0.000), and day 7 (P = 0.000) after admission. FDP was significantly higher in patients with POF than in those without on day 1 (P = 0.000), day 2 (P = 0.000), day 3 (P = 0.000), and day 7 (P = 0.000). Patients with PN had signficantly higher FDP on day 2 (P = 0.021), day 3 (P = 0.000), and day 7 (P = 0.000) after admission. FIB did not differ significantly between AP patients with POF and those without, or between AP patients with PN and those without. ROC analysis revealed that D-D (AUC = 0.693) and FDP (AUC = 0.711) were superior to CRP (AUC = 0.615) and LDH (AUC = 0.672) in predicting POF on day 1 of hospital admission, and D-D (AUC = 0.832) and FDP (AUC = 0.814) were superior than LDH (AUC = 0.639) and CRP (AUC = 0.706) in predicting PN on day 3 of hospital admission.
Plasma FRMs in AP patients increase significantly on the first week after admission. FDP and D-D correlate with disease severity of AP and can be considered as a potentially useful tool for the early diagnosis of AP with POF and PN.
- Citation: Lei JJ, Zhou L, Xiong C, Liu Q, Deng WH. Clinical utility of fibrin-related biomarkers in human acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2018; 26(19): 1176-1185
- URL: https://www.wjgnet.com/1009-3079/full/v26/i19/1176.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v26.i19.1176
纤维蛋白相关标志物(fibrin-related markers, FRMs)是包括纤维蛋白原(fibrinogen, FIB)、纤维蛋白(fibrin, Fib)及其活化产物-可溶性纤维蛋白单体(fibrin monomer, FM), 以及D-二聚体(D-Dimer, D-D)和纤维蛋白(原)降解产物(fibrinogen and fibrin degradation products, FDP)在内的蛋白或片段, 是体内凝血活化和纤溶亢进的标志, 由于其在体内血液凝固通路中所处的位置不同, 其改变代表的临床意义也不同. FM是血液凝固途径上游标志物, 含量增加提示凝血酶生成增加, 是疾病进程中凝血系统被激活的敏感指标[1,2], 是血栓即将发生的标志, 又被称为血栓前标志物[3,4]. FDP和D-D是纤维蛋白血栓被纤溶酶降解的产物, 是一种血栓后的标志物, 不仅代表凝血系统的活化, 还代表纤溶系统的活化, 被称为血栓后标志物[5,6]. FIB是形成FM、FDP和D-D的前体物质, 由肝细胞和内皮细胞合成, 在炎症状态下可能增高2-3倍, FIB增高提示机体处于高凝状态[7]. 在正常生理状态下, 体内凝血、抗凝和纤溶系统处于动态的平衡中, 这些分子标志物的血浆含量稳定在一定范围.
研究急性胰腺炎(acute pancreatitis, AP)时凝血和纤溶指标的变化的报道较多, 且有文献报道D-D预测重症胰腺炎的AUC大于0.9, 敏感性高达90%以上[8,9], 与Gravante等[10]对58篇文献的分析和临床上观测到的单一的血清标志物预测AP严重性价值有限的观点不一致, 为了帮助临床医生更好的理解FRMs 在AP病人血浆中的变化和临床意义, 我们监测了入院后第1、2、3和7天AP病人的FRMs的变化, 对其预测AP早期发生POF和PN的价值进行了评估, 报道如下.
在这个前瞻性的观察试验中, 我们收录了从2015-04/2016-12入住贵州医科大学附属白云医院消化内科的152个AP病人, 男92例, 平均年龄46.73岁±12.90岁, 女60例, 平均年龄48.92岁±16.92岁, 男女AP病人年龄差异无显著性(t = -0.871, P = 0.386). 所有病人均是第1次因AP住院, 症状发生到入院时间小于24 h, 需要外科手术的胆源性AP被退出研究, ERCP和药物诱导的AP不包括在内, 病例的纳入标准和AP的诊断标准见已发表文献[11]. AP的严重性分类采用2012年修订的亚特兰大标准[12], 按照有无持续器官功能衰竭(persistent organ failure, POF)分为无POF组120例和POF组32例(图1A). 按照有无胰腺坏死(pancreatic necrosis, PN)分为PN组44例, 无PN组108例(图1B). 贵州医科大学附属白云医院学术委员会批准本临床研究, 入选病例征得病人本人和家属的同意并签署了知情同意书.
患者入院后第1(入院当时)、2、3和7天(清晨空腹)抽血查血常规、肝肾功电解质、血淀粉酶、DIC全套、C-反应蛋白(C-reaction protein, CRP)、抗凝血酶Ⅲ(antithrombin-Ⅲ, AT-Ⅲ)和FM. 为减少血标本放置过久和抗凝剂对FRMs测定的影响, 分离血浆测定FRMs水平的血标本采用3.2%枸橼酸钠抗凝, 并在采血后立即送检. 血浆FRMs水平测定采用STAGO-R EVOLUTION全自动血凝分析仪(免疫比浊法, 法国Stago公司). 血浆FM参考值: 0-6 μg/mL; D-D 参考值: 0.22-4 μg/mL; FDP参考值0-5 μg/mL; FIB参考值2-4 g/L, 并在入院第3天后做上腹增强CT了解有无PN.
统计学处理 采用EXCEL表收集资料, SPSS21.0统计软件包进行统计分析. 正态分布的连续变量用均数±标准差表示, 两组间均数比较采用独立样本t检验与Mann-Whitney U检验. 偏态分布的连续变量用中位数 (Q1,Q3) 表示, 两组间比较采用Mann-Whitney t检验或者Two-Sample Kolmogorov-Smirnov检验. 卡方检验用于其他的分类变量. P<0.05定义为差异具有统计学意义. ROC曲线分析用于比较FM、FDP和D-D等血清标志物预测POF和PN的敏感性, 特异性, 阳性预测值和阴性预测值, 曲线下面积的比较采用正态性Z检验, 设定P<0.05为差异具有统计学意义.
结果发现无论是按照有无POF分组, 还是按照有无PN分组, 年龄、性别和病因构成比在不同严重程度的AP中差异无显著性(P˃0.05), 转氨酶(ALT和AST)、肾功能(BUN和Cr)、电解质(Ca2+和Na+)和AMY在不同严重程度的AP中差异无显著性(P˃0.05). 二种分组的SAP中均明显增高的是TB和Glu, ALB在POF组和PN组均减低, 在POF组和无POF组间差异有显著性. 乳酸脱氢酶(lactate dehydrogenase, LDH)在POF组和PN组明显高于无POF和无PN组. CRP在有无POF组和有无PN组间差异无显著性(表1和表2).
| 变量 | POF组 (n = 32) | 无POF组 (n = 120) | t (χ2) | P |
| 年龄 | 50.28 ± 14.13 | 46.88 ± 14.43 | -1.191 | 0.2351 |
| 性别 (男/女) | 24/8 (75.0%) | 68/52 (56.7%) | 3.554 | 0.0592 |
| 病因 | ||||
| 高脂血症 | 12/65 (18.5%) | 53/65 (81.5%) | 0.47 | 0.9252 |
| 胆源性 | 13/57 (22.8%) | 44/57 (77.2%) | ||
| 酗酒 | 1/4 (25.0%) | 3/4 (75%) | ||
| 不明原因 | 6/26 (23.1%) | 20/26 (76.9%) | ||
| ALT (U/L) | 97.39 ± 169.14 | 74.56 ± 127.42 | 0.822 | 0.4121 |
| AST (U/L) | 73.57 ± 92.08 | 67.04 ± 118.20 | 0.281 | 0.7791 |
| 总胆 (mg/dL) | 29.08 ± 34.31 | 16.25 ± 11.81 | 2.05 | 0.0491 |
| 直胆 (mg/dL) | 15.58 ± 27.91 | 6.04 ± 7.75 | 1.914 | 0.0651 |
| LDH (U/L) | 283.87 ± 82.20 | 239.43 ± 92.45 | 2.369 | 0.0191 |
| 淀粉酶 (U/L) | 590.18 ± 572.56 | 635.85 ± 1010.81 | -0.229 | 0.8191 |
| 血糖 (mg/dL) | 11.26 ± 4.77 | 8.17 ± 3.99 | 3.714 | 0.0001 |
| BUN (mg/dL) | 4.70 ± 2.24 | 6.74 ± 12.95 | -0.884 | 0.3781 |
| Cr (mg/dL) | 68.19 ± 27.43 | 61.87 ± 23.81 | 1.287 | 0.2001 |
| 白蛋白 (g/L) | 39.67 ± 9.16 | 44.73 ± 7.60 | -3.188 | 0.0021 |
| 钙 (mg/dL) | 2.54 ± 1.54 | 2.29 ± 0.40 | 0.86 | 0.3971 |
| 钠 (mmol/L) | 137.31 ± 5.22 | 138.19 ± 4.78 | -0.877 | 0.3821 |
| CRP (mg/L) | 76.03 ± 88.97 | 46.70 ± 75.41 | 1.874 | 0.0631 |
| 变量 | PN组 (n = 44) | 无PN组 (n = 108) | t (χ2) | P |
| 年龄 (岁) | 47.64 ± 13.26 | 47.57 ± 14.89 | 0.024 | 0.9811 |
| 性别 (男/女) | 26/18(59.1%) | 66/42(61.1%) | 0.053 | 0.8172 |
| 病因 | 0.660 | 0.8822 | ||
| 高脂血症 | 19/65(29.2%) | 46/65(70.8%) | ||
| 胆源性 | 18/57(31.6%) | 39/57(68.4%) | ||
| 酗酒 | 1/4(25.0%) | 3/4(75.0%) | ||
| 不明原因 | 6/26(23.1%) | 20/26(76.9%) | ||
| ALT (U/L) | 84.78 ± 152.65 | 77.17 ± 130.67 | 0.305 | 0.7611 |
| AST (U/L) | 69.61 ± 98.77 | 67.87 ± 118.98 | 0.084 | 0.9331 |
| TB (μmol/L) | 26.82 ± 31.68 | 15.70 ± 9.54 | 2.260 | 0.0291 |
| DB (μmol/L) | 14.17 ± 25.73 | 5.61 ± 5.57 | 2.160 | 0.0361 |
| LDH (U/L) | 274.62 ± 92.29 | 237.30 ± 90.06 | 2.272 | 0.0251 |
| AMY (U/L) | 750.33 ± 705.14 | 579.00 ± 1005.02 | 0.938 | 0.3501 |
| GLu (mmol/L) | 10.13 ± 5.19 | 8.29 ± 3.83 | 2.406 | 0.0171 |
| BUN (mmol/L) | 4.90 ± 3.22 | 6.87 ± 13.52 | -0.941 | 0.3481 |
| Cr (μmol/L) | 64.85 ± 32.33 | 62.54 ± 20.81 | 0.519 | 0.6051 |
| ALB (g/L) | 41.71 ± 8.75 | 44.45 ± 7.85 | -1.877 | 0.0621 |
| Ca2+ (mmol/L) | 2.32 ± 0.69 | 2.35 ± 0.81 | -0.233 | 0.8161 |
| Na+ (mmol/L) | 136.98 ± 6.18 | 138.45 ± 4.16 | -1.684 | 0.0941 |
| CRP (mg/L) | 64.03 ± 94.06 | 48.52 ± 72.23 | 1.084 | 0.2801 |
入院后1 wk, FM在有无POF组间差异无显著性. 入院后1 wk, POF组的D-D和FDP明显高于无POF组. 入院后1 wk, POF组FIB高于无POF组, 但只在入院后第7天差异才有显著性(表3).
| FM | POF组 | 无POF组 | Z (t) | P |
| FM1 | 5.12 (5.00, 13.25) | 5.49 (5.00, 8.02) | -0.290 | 0.7711 |
| FM2 | 6.80 (5.00, 14.35) | 5.22 (5.00, 6.98) | -1.854 | 0.0641 |
| FM3 | 5.90 (5.00, 7.21) | 5.17 (5.00, 7.60) | -0.643 | 0.5201 |
| FM7 | 6.90 (5.00, 12.11) | 5.96 (5.00, 8.31) | -0.926 | 0.3541 |
| D-D1 | 1.41 (0.66, 4.00) | 0.67 (0.39, 1.15) | -3.247 | 0.0011 |
| D-D2 | 2.79 (1.39, 4.00) | 1.43 (0.69, 2.48) | -2.911 | 0.0041 |
| D-D3 | 4.00 (2.51, 4.00) | 1.38 (0.82, 2.75) | -4.353 | 0.0001 |
| D-D7 | 4.00 (2.01, 4.00) | 1.90 (0.81, 3.36) | -3.060 | 0.0021 |
| FDP1 | 7.26 (3.01, 25.84) | 3.07 (1.73, 5.46) | -3.497 | 0.0001 |
| FDP2 | 16.95 (5.20, 27.15) | 4.49 (3.23, 8.82) | -3.158 | 0.0021 |
| FDP3 | 22.15 (8.66, 27.78) | 6.26 (3.47, 13.48) | -4.223 | 0.0001 |
| FDP7 | 15.93 (8.33, 31.63) | 5.38 (2.91, 11.40) | -3.753 | 0.0001 |
| FIB1 | 3.91 ± 1.95 | 3.70 ± 1.33 | 0.564 | 0.5762 |
| FIB2 | 5.45 ± 2.38 | 4.72 ± 1.45 | 1.211 | 0.2332 |
| FIB3 | 5.57 ± 1.93 | 4.96 ± 1.98 | 1.426 | 0.1572 |
| FIB7 | 5.04 ± 1.69 | 4.29 ± 1.27 | 2.300 | 0.0242 |
入院后1 wk, FM在PN组明显高于无PN组, 差异有显著性, 入院后1 wk, D-D在PN组明显高于无PN组, 入院后第2、3和7天PN组的FDP高于无PN组. 有无PN组的FIB在入院后1 wk差异均无显著性(表4).
| FM | PN组 (44) | 无PN组 (108) | Z (t) | P |
| FM1 | 6.82 (5.00, 18.09) | 5.00 (5.00, 7.69) | -2.026 | 0.0431 |
| FM2 | 6.47 (5.00, 12.58) | 5.00 (5.00, 6.98) | -2.650 | 0.0081 |
| FM3 | 7.11 (5.00, 13.09) | 5.00 (5.00, 6.93) | -3.277 | 0.0011 |
| FM7 | 7.50 (5.91, 10.68) | 5.52 (5.00, 7.60) | -3.030 | 0.0041 |
| D-D1 | 0.97 (0.63, 1.83) | 0.66 (0.39, 1.30) | -2.276 | 0.0231 |
| D-D2 | 1.79 (1.28, 4.00) | 1.42 (0.73, 3.56) | -1.163 | 0.0451 |
| D-D3 | 4.00 (2.66, 4.00) | 1.30 (0.75, 2.49) | -5.676 | 0.0001 |
| D-D7 | 4.00 (2.66, 4.00) | 1.57 (0.88, 2.93) | -4.115 | 0.0001 |
| FDP1 | 3.73 (2.56, 8.41) | 3.14 (1.72, 6.17) | -1.803 | 0.0711 |
| FDP2 | 9.38 (4.06, 22.24) | 4.68 (3.11, 9.85) | -2.324 | 0.0211 |
| FDP3 | 20.69 (10.14, 27.65) | 5.74 (3.31, 11.26) | -5.392 | 0.0001 |
| FDP7 | 17.34 (7.57, 30.90) | 4.81 (2.79, 8.42) | -5.207 | 0.0001 |
| FIB1 | 3.63 ± 1.62 | 3.80 ± 1.43 | -0.649 | 0.5172 |
| FIB2 | 4.82 ± 1.98 | 5.02 ± 1.6 | -0.353 | 0.7262 |
| FIB3 | 5.46 ± 2.16 | 4.95 ± 1.88 | 1.220 | 0.2272 |
| FIB7 | 4.59 ± 1.64 | 4.42 ± 1.32 | 0.545 | 0.5872 |
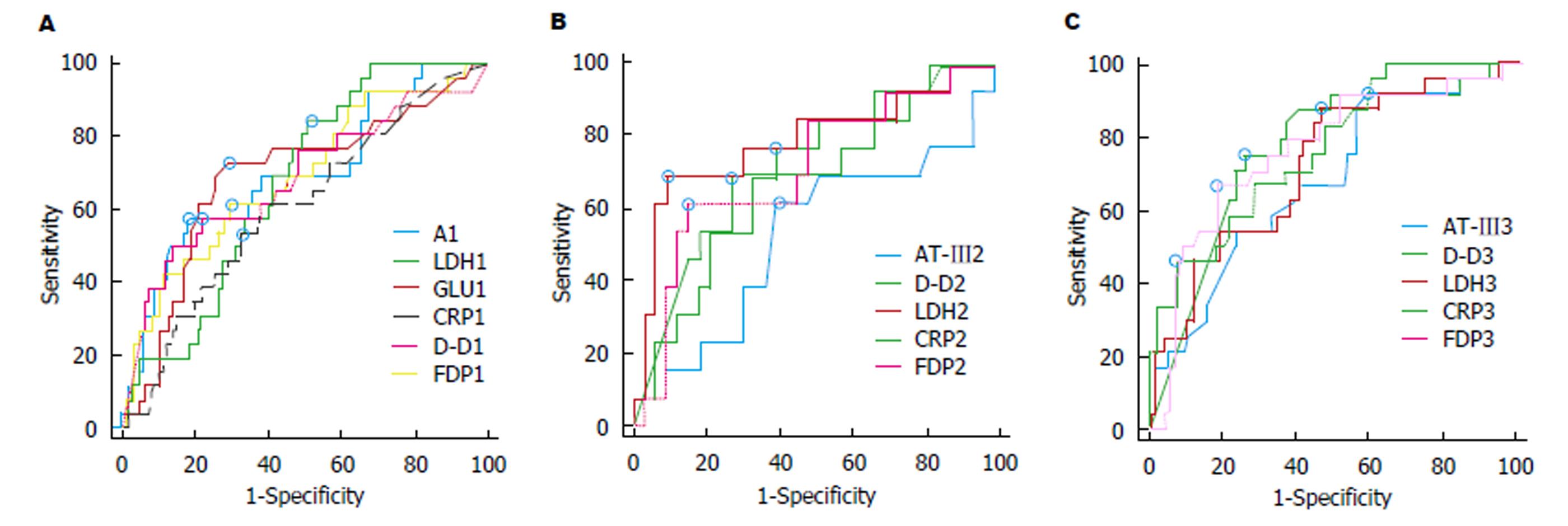
因入院后1 wk FM、FIB在有无POF的患者中表现并无差异性, 故我们对有差异性的D-D、FDP作ROC曲线进一步评价其预测POF的价值, 并与临床上常用的评价指标ALB[13,14], Glu[15,16], LDH[17,18]和CRP[19,20]作一比较, 结果发现上述指标对POF均有一定的预测价值(P<0.05), 但入院后第1天AUC>0.7的指标只有FDP和Glu, 入院后第2、3天FDP、LDH、D-D、CRP预测POF的效果相当, AUC均>0.7. LDH在第2天预测效果较好, 而CRP在入院后第3天最好(表5和图2).
| Cut-offvalue | AUC | 95%CI | Sn (%) | Sp (%) | PPV (%) | NPV (%) | Z | P | |
| D-D1 | 1.18 | 0.693 | (0.611, 0.767) | 60.00 | 76.99 | 40.91 | 87.88 | 3.247 | 0.0012 |
| FDP1 | 4.63 | 0.711 | (0.629, 0.784) | 65.52 | 70.80 | 36.53 | 88.89 | 3.725 | 0.0002 |
| LDH1 | 218.72 | 0.672 | (0.590, 0.747) | 82.76 | 49.58 | 28.57 | 92.19 | 3.528 | 0.0004 |
| GLU1 | 8.6 | 0.720 | (0.640, 0.790) | 71.87 | 75.21 | 42.24 | 90.72 | 3.913 | 0.0001 |
| A1 | 40.58 | 0.661 | (0.579, 0.737) | 53.13 | 81.90 | 44.74 | 86.37 | 2.725 | 0.0064 |
| CRP1 | 84 | 0.615 | (0.532, 0.694) | 34.38 | 86.32 | 40.74 | 82.78 | 2.022 | 0.0431 |
| D-D2 | 2.07 | 0.731 | (0.604, 0.836) | 73.68 | 74.42 | 56.01 | 86.48 | 3.357 | 0.0008 |
| FDP2 | 10.69 | 0.755 | (0.632, 0.854) | 61.11 | 82.61 | 57.89 | 84.21 | 3.881 | 0.0001 |
| AT-Ⅲ2 | 55 | 0.584 | (0.498, 0.666) | 25.00 | 98.25 | 77.82 | 84.21 | 1.330 | 0.1836 |
| LDH2 | 261.59 | 0.761 | (0.684, 0.828) | 54.84 | 87.93 | 54.84 | 87.93 | 5.156 | <0.0001 |
| CRP2 | 74.05 | 0.718 | (0.684, 0.828) | 83.33 | 51.72 | 30.86 | 92.30 | 4.446 | <0.0001 |
| D-D3 | 1.94 | 0.776 | (0.687, 0.849) | 88.90 | 61.90 | 42.85 | 94.55 | 5.651 | <0.0001 |
| FDP3 | 15.51 | 0.769 | (0.682, 0.843) | 66.67 | 80.68 | 51.43 | 88.75 | 4.997 | <0.0001 |
| AT-Ⅲ3 | 95 | 0.664 | (0.581, 0.740) | 93.33 | 37.39 | 28.00 | 95.55 | 2.930 | 0.0034 |
| LDH3 | 233.64 | 0.731 | (0.651, 0.801) | 55.17 | 82.91 | 44.45 | 88.18 | 4.363 | <0.0001 |
| CRP3 | 203 | 0.777 | (0.506, 0.680) | 45.16 | 95.65 | 73.67 | 86.62 | 6.151 | <0.0001 |
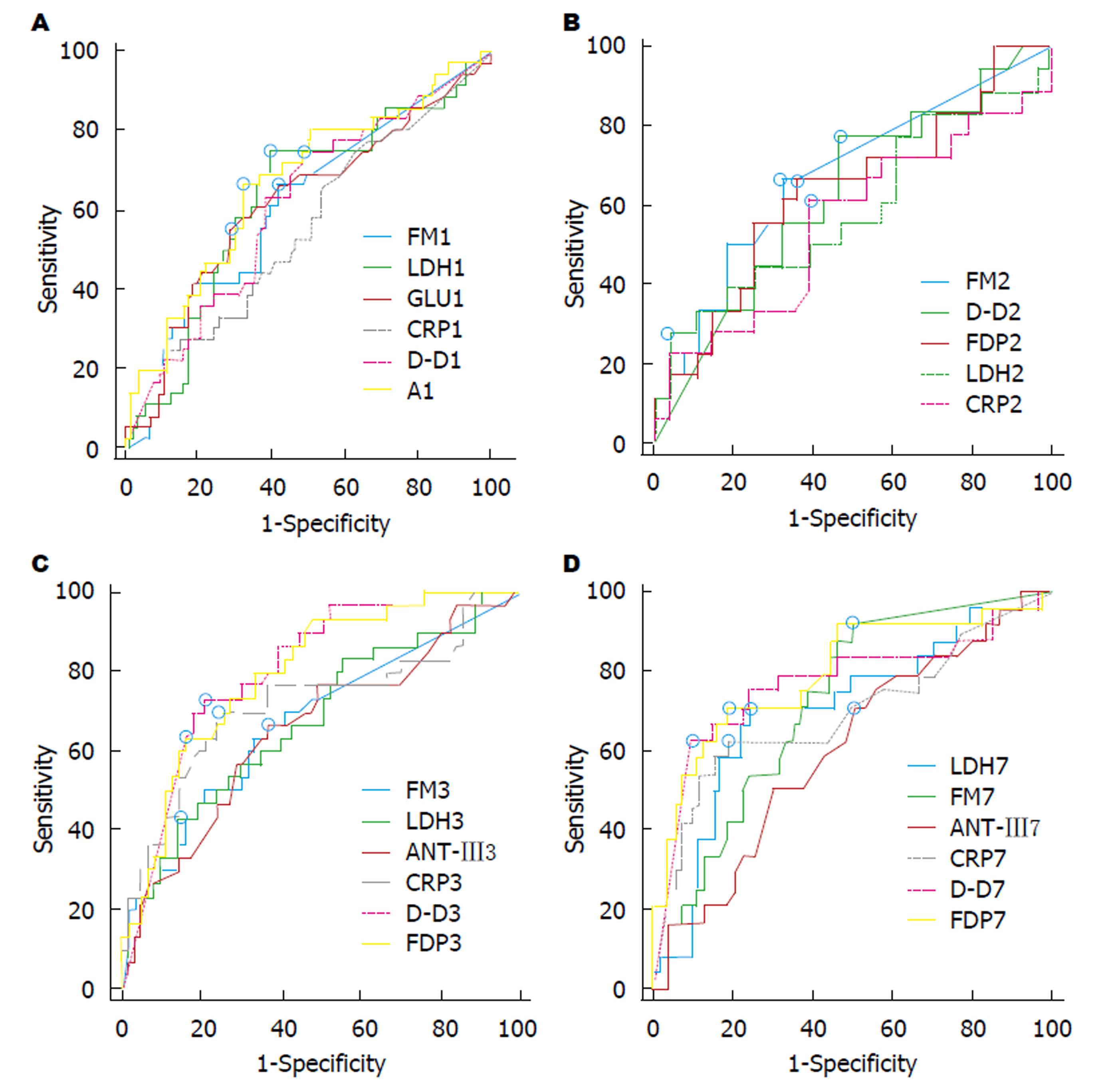
ROC曲线分析表明, 入院后第1天, 对PN有预测价值的指标有D-D, Glu, LDH和Alb, 入院后第2天, 对PN有预测价值的指标FDP、FM、LDH和CRP, 但AUC均<0.7; 入院后第3天D-D和FDP预测PN的AUC分别为0.832和0.814, 明显优于CRP和LDH. 入院后第7天FM、D-D、FDP和AT-Ⅲ, LDH和CRP对AP伴PN均有中等程度的预测价值, 但FDP预测价值最高, AUC为0.825(表6和图3).
| Cut-offvalue | AUC | 95%CI | Sn (%) | Sp (%) | PPV (%) | NPV (%) | Z | P | |
| FM1 | 5.58 | 0.595 | (0.506, 0.680) | 62.50 | 58.70 | 39.68 | 78.26 | 1.810 | 0.0703 |
| D-D1 | 0.86 | 0.622 | (0.537, 0.702) | 63.41 | 62.75 | 40.62 | 81.01 | 2.354 | 0.0186 |
| LDH1 | 240.66 | 0.635 | (0.552, 0.712) | 66.44 | 64.76 | 43.93 | 82.93 | 2.620 | 0.0088 |
| GLU1 | 8.2 | 0.629 | (0.546, 0.706) | 56.82 | 68.57 | 40.68 | 79.42 | 2.483 | 0.0130 |
| ALB1 | 43.15 | 0.607 | (0.523,0.686) | 61.36 | 62.50 | 40.91 | 79.27 | 2.064 | 0.0390 |
| CRP1 | 78 | 0.545 | (0.462, 0.627) | 27.91 | 83.96 | 41.38 | 74.17 | 0.854 | 0.3931 |
| FM2 | 5.94 | 0.639 | (0.549, 0.723) | 60.98 | 69.41 | 49.02 | 78.67 | 2.711 | 0.0067 |
| D-D2 | 1.42 | 0.590 | (0.458, 0.713) | 76.19 | 51.22 | 44.44 | 80.77 | 1.212 | 0.2256 |
| FDP2 | 6.23 | 0.676 | (0.548, 0.788) | 68.18 | 66.67 | 51.73 | 80.00 | 2.489 | 0.0128 |
| LDH2 | 278.31 | 0.633 | (0.550, 0.711) | 31.82 | 91.26 | 60.86 | 75.81 | 2.569 | 0.0102 |
| CRP2 | 102 | 0.614 | (0.530, 0.694) | 53.49 | 68.93 | 41.81 | 78.02 | 2.186 | 0.0288 |
| FM3 | 5.87 | 0.683 | (0.590, 0.766) | 65.71 | 67.50 | 46.93 | 81.82 | 3.339 | 0.0008 |
| D-D3 | 2.63 | 0.832 | (0.749, 0.896) | 77.14 | 81.58 | 65.85 | 88.57 | 8.422 | <0.0001 |
| FDP3 | 14.09 | 0.814 | (0.731, 0.881) | 69.44 | 83.54 | 65.78 | 85.71 | 7.502 | <0.0001 |
| AT-Ⅲ3 | 81 | 0.635 | (0.551, 0.713) | 59.09 | 68.32 | 44.82 | 79.31 | 2.613 | 0.0090 |
| LDH3 | 160.89 | 0.639 | (0.556, 0.717) | 88.10 | 37.50 | 36.27 | 88.64 | 2.754 | 0.0059 |
| CRP3 | 105.03 | 0.706 | (0.625, 0.779) | 65.91 | 76.47 | 54.72 | 83.37 | 4.072 | <0.0001 |
| FM7 | 5.87 | 0.698 | (0.596, 0.788) | 88.46 | 52.17 | 41.07 | 92.31 | 3.574 | 0.0004 |
| D-D7 | 3.87 | 0.761 | (0.664, 0.841) | 65.52 | 86.96 | 67.86 | 85.72 | 4.371 | <0.0001 |
| FDP7 | 8.79 | 0.825 | (0.735, 0.894) | 75.00 | 82.09 | 66.66 | 87.30 | 6.610 | <0.0001 |
| AT-Ⅲ7 | 94 | 0.639 | (0.552, 0.720) | 78.05 | 45.26 | 38.10 | 82.69 | 2.679 | 0.0074 |
| LDH7 | 177.12 | 0.661 | (0.575, 0.740) | 78.57 | 50.00 | 41.25 | 83.93 | 3.182 | 0.0015 |
| CRP7 | 42 | 0.708 | (0.624, 0.783) | 60.98 | 81.91 | 59.52 | 82.80 | 3.934 | 0.0001 |
关于AP时FM的研究报道最早见于1990年Lindahl等[21]对癌症病人和急性炎症病人的研究, 提示AP时FM是增高的, 但研究的例数较少, 只有4例AP病人而且无AP严重程度的描述, 由于FM的半衰期只有2.3 h, 仅来源于血管内, FM被认为是体内凝血活化最敏感的指标, 而且与炎症反应和纤溶活化无关, 我们的研究中FM在AP病人中明显增高.
关于FM与PN的研究未见报道, 我们的研究表明PN组的FM高于无PN组, 但FM在有无POF的AP组间差异并不显著, 提示AP时发生POF除了与凝血活化相关外, 更与个体对凝血活化产生的炎症反应相关, 有全身炎症反应者更可能发生多器官功能的衰竭[22].
四种FRMs中D-D对AP严重性的预测研究报道最多, Radenkovic等[8]报道入院时D-D预测OF的敏感性为90%、特异性为89%、AUC = 0.908, 入院后24 h D-D预测OF的敏感性为90%、特异性为81%、AUC = 0.916, 明显高于我们研究中入院第1天D-D预测POF的敏感性60.00%、特异性76.99%和AUC = 0.693, 入院后第2天预测POF的敏感性73.68%、特异性74.42%和AUC = 0.731. 我们的研究与Badhal等[23]报道的敏感性81.7、特异性54.2%, AUC = 0.683类似, 说明D-D预测POF只有中等程度的预测价值, 并不优于入院时的LDH, 敏感性为82.76%, AUC = 0.672和入院第二天的CRP, 敏感性为83.33, AUC = 0.718. 对于D-D对PN预测研究未见报道, 我们的研究表明入院后第3天D-D预测PN的敏感性、特异性分别为77.14%、81.58%, AUC = 0.832, 明显优于LDH和CRP, 与Boskovic等[24]的研究相近. 我们的研究中发现D-D在入院后第7天继续增高, 无论在轻症还是重症, 其原因和意义有待进一步阐述.
FDP是在纤溶酶作用下, FIB发生降解产生X, Y, D, E碎片(FgDP)和纤维蛋白发生降解产生X', Y', D', E'(FbDP)的总称. FDP的升高可以来源于血管内和或血管外纤维蛋白原-纤维蛋白的降解. 临床上主要与D-D联合检测来区别原发和继发的纤溶. 关于FDP预测AP的严重性的研究有限, Maeda等[25]报道入院时FDP-E˃894 ng/mL预测AP伴死亡的敏感性、特异性分别为93%, 73%, AUC = 0.873, 多数研究认为FDP在AP中是增高的[26,27], 与我们的研究相同, 但未对FDP预测AP伴POF和PN作进一步的分析, 我们的研究表明入院后第1天AUC>0.7的指标只有FDP和血糖, 提示FDP预测POF可能优于D-D, 而入院后第3天FDP预测PN的敏感性为69.44%, 特异性为83.54%, AUC为0.814, 提示FDP对AP严重性的判断与D-D的价值相近.
FIB是血浆中含量最高的凝血因子, 在正常人血浆浓度是2.0-4.0 g/L, 其在血中的浓度受酶解的速度和生成的速度的影响, 如生成速度下降或酶解速度加快, 血浆中FIB就下降, 如生成速度加快或酶解速度变慢, FIB就升高. 我们的研究表明AP病人在入院后1 wk的FIB均明显高于对照组, 但FIB的增高在有无POF组间差异不显著, 在有无PN的病人中差异也不显著, 提示AP病程中FIB增高与急性炎症时肝脏合成FIB增高有关, 与国内外的研究就AP时FIB是增高的是吻合的[28].
总之, 我们的研究表明AP时4种FRMs均是增高的. 其中FDP和D-D的增高可持续1 wk, FDP和D-D对POF的预测在入院后第1天优于CRP和LDH, 而对PN的预测在入院后第3天较好, 优于常用的CRP和LDH. 但与其他预测AP伴POF的指标一样, FDP和D-D对POF和PN均有一定程度的预测价值 (AUC均大于0.7), 但并不能作为AP伴发POF和PN的预测指标(一个好的诊断试验其AUC应大于0.9), 只能作为AP严重性判断的辅助指标.
关于急性胰腺炎(acute pancreatitis, AP)时凝血和纤溶相关指标的研究文献较多, 但多数是对作为血栓后标志物D-二聚体(D-Dimer, D-D)的研究, 对作为纤溶亢进指标的纤维蛋白降解产物(fibrin degradation products, FDP)的研究较少, 而对于血栓前标志物的纤维蛋白单体(fibrin monomer, FM)的研究罕有报道.
AP时凝血和纤溶系统的活化是AP严重性的表现, 纤维蛋白相关标志物在AP发病的不同时期有什么样的变化?该变化在多大程度上反映了AP的严重性?
我们采用FM代表凝血活化, D-D和FDP代表血栓形成和纤溶亢进, 纤维蛋白原(fibrinogen, FIB)代表血液的高凝状态, 研究入院后1 wk的AP的4种纤维蛋白单体的变化, 及该变化与AP严重性的关系.
我们采用了前瞻性的研究方法, 动态观察不同严重程度AP的4种FRMs在入院后1 wk的变化, 并且通过ROC曲线分析, 对FRMs和C-反应蛋白(C-reaction protein, CRP)、乳酸脱氢酶(lactate dehydrogenase, LDH)等血清标志物对胰腺坏死和POF的预测价值进行了比较
AP发病1 wk内4种纤维蛋白相关标志物均增高, AP中FDP和D-D的增高可持续1 wk, 入院后1 wk D-D和FDP在POF组高于无POF组, PN组FM、D-D、FDP高于无PN组. 入院第3天D-D和FDP预测PN的AUC分别为0.832和0.814, 明显优于CRP和LDH.
AP病人在发病的早期均有凝血和纤溶系统的活化, FIB与AP的严重性无关, FM持续增高与PN相关, D-D和FDP与AP的严重性明显相关; 对于预测POF和PN来说, D-D和FDP只有辅助价值.
本课题的不足在于研究周期短, 是一个单中心的研究, 入组病例数较少, 没有按照病因分类, 未来的研究是对不同病因, 尤其是高脂血症AP的FRMs的变化进行研究.
学科分类: 胃肠病学和肝病学
手稿来源地: 贵州省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C
D级 (一般): D, D
E级 (差): 0
编辑: 马亚娟 电编:张砚梁
| 1. | Toh JM, Ken-Dror G, Downey C, Abrams ST. The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis. 2013;24:839-843. [PubMed] [DOI] |
| 2. | Takahashi D, Takahashi Y, Matsui M, Araki S, Kubo K, Sato H, Shirahata A. Evaluation of hypercoagulability using soluble fibrin monomer complex in sick newborns. Pediatr Int. 2013;55:151-156. [PubMed] [DOI] |
| 3. | Wada H, Hatada T, Okamoto K, Uchiyama T, Kawasugi K, Mayumi T, Gando S, Kushimoto S, Seki Y, Madoiwa S. Modified non-overt DIC diagnostic criteria predict the early phase of overt-DIC. Am J Hematol. 2010;85:691-694. [PubMed] [DOI] |
| 4. | Wada H, Kobayashi T, Abe Y, Hatada T, Yamada N, Sudo A, Uchida A, Nobori T. Elevated levels of soluble fibrin or D-dimer indicate high risk of thrombosis. J Thromb Haemost. 2006;4:1253-1258. [PubMed] [DOI] |
| 5. | Gaffney PJ. Fibrin degradation products. A review of structures found in vitro and in vivo. Ann N Y Acad Sci. 2001;936:594-610. [PubMed] |
| 6. | Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011;31:494-499. [PubMed] [DOI] |
| 7. | Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb. 1994;14:54-59. [PubMed] |
| 8. | Radenkovic D, Bajec D, Ivancevic N, Milic N, Bumbasirevic V, Jeremic V, Djukic V, Stefanovic B, Stefanovic B, Milosevic-Zbutega G. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas. 2009;38:655-660. [PubMed] [DOI] |
| 9. | Ke L, Ni HB, Tong ZH, Li WQ, Li N, Li JS. D-dimer as a marker of severity in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2012;19:259-265. [PubMed] [DOI] |
| 10. | Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, Dennison AR. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9:601-614. [PubMed] [DOI] |
| 11. | Lei JJ, Zhou L, Liu Q, Xiong C, Xu CF. Can mean platelet volume play a role in evaluating the severity of acute pancreatitis? World J Gastroenterol. 2017;23:2404-2413. [PubMed] [DOI] |
| 12. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [PubMed] [DOI] |
| 13. | Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340-1346. [PubMed] |
| 14. | Lankisch PG, Blum T, Bruns A, Dröge M, Brinkmann G, Struckmann K, Nauck M, Maisonneuve P, Lowenfels AB. Has blood glucose level measured on admission to hospital in a patient with acute pancreatitis any prognostic value? Pancreatology. 2001;1:224-229. [PubMed] [DOI] |
| 15. | Rajaratnam SG, Martin IG. Admission serum glucose level: an accurate predictor of outcome in gallstone pancreatitis. Pancreas. 2006;33:27-30. [PubMed] [DOI] |
| 16. | Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit VK. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013;2013:367581. [PubMed] [DOI] |
| 17. | Zrnić IK, Milić S, Fisić E, Radić M, Stimac D. [C-reactive protein and lactate dehydrogenase as single prognostic factors of severity in acute pancreatitis]. Lijec Vjesn. 2007;129:1-4. [PubMed] |
| 18. | Puolakkainen P, Valtonen V, Paananen A, Schröder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987;28:764-771. [PubMed] |
| 19. | Pongprasobchai S, Jianjaroonwong V, Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S, Pausawasdi N, Srikureja W, Chainuvati S, Prachayakul V. Erythrocyte sedimentation rate and C-reactive protein for the prediction of severity of acute pancreatitis. Pancreas. 2010;39:1226-1230. [PubMed] [DOI] |
| 20. | Tasić T, Grgov S, Nagorni A, Benedeto-Stojanov D. [Comparison of biohumoral and morphological parameters in acute pancreatitis]. Srp Arh Celok Lek. 2014;142:29-33. [PubMed] |
| 21. | Lindahl AK, Sandset PM, Abildgaard U. Indices of hyperco-agulation in cancer as compared with those in acute inflammation and acute infarction. Haemostasis. 1990;20:253-262. [PubMed] |
| 22. | Dumnicka P, Maduzia D, Ceranowicz P, Olszanecki R, Drożdż R, Kuśnierz-Cabala B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int J Mol Sci. 2017;18. [PubMed] [DOI] |
| 23. | Badhal SS, Sharma S, Saraya A, Mukhopadhyay AK. Prognostic significance of D-dimer, natural anticoagulants and routine coagulation parameters in acute pancreatitis. Trop Gastroenterol. 2012;33:193-199. [PubMed] |
| 24. | Boskovic A, Pasic S, Soldatovic I, Milinic N, Stankovic I. The role of D-dimer in prediction of the course and outcome in pediatric acute pancreatitis. Pancreatology. 2014;14:330-334. [PubMed] [DOI] |
| 25. | Maeda K, Hirota M, Ichihara A, Ohmuraya M, Hashimoto D, Sugita H, Takamori H, Kanemitsu K, Baba H. Applicability of disseminated intravascular coagulation parameters in the assessment of the severity of acute pancreatitis. Pancreas. 2006;32:87-92. [PubMed] |
| 26. | Berry AR, Taylor TV, Davies GC. Pulmonary function and fibrinogen metabolism in acute pancreatitis. Br J Surg. 1981;68:870-873. [PubMed] |
| 27. | Atomi Y, Ohnishi H, Watanabe C, Ishiyama M, Kuroda A, Morioka Y. [Pathophysiology and prognosis of acute pancreatitis--early and late prognostic signs]. Nihon Geka Gakkai Zasshi. 1985;86:1257-1260. [PubMed] |
| 28. | Andersson E, Axelsson J, Eckerwall G, Ansari D, Andersson R. Tissue factor in predicted severe acute pancreatitis. World J Gastroenterol. 2010;16:6128-6134. [PubMed] |