修回日期: 2018-03-28
接受日期: 2018-04-04
在线出版日期: 2018-04-28
探究慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)合并肝肾综合征(hepatorenal syndrome, HRS)患者的胱抑素C(cystatin C, Cys-C)、β2微球蛋白(microglobulin, β2-MG)、血肌酐(serum creatinine, Scr)和血尿素氮(blood urea nitrogen, BUN)水平及其临床价值分析.
对2014-02/2017-12于绍兴市中心医院就诊的36例ACLF合并HRS患者(HRS组)进行回顾性分析, 选择同时间段内36例单纯ACLF患者(ACLF组)和50例慢性肝病患者(CLD组)作为对照组, 比较3组的Cys-C、β2-MG、Scr和BUN水平等临床资料间差异, 应用受试者工作曲线(receiver operating characteristic curves, ROC)评价应用Cys-C、β2-MG、Scr和BUN水平预测ACLF合并HRS的价值, 并计算4种指标单独和联合预测的诊断效能.
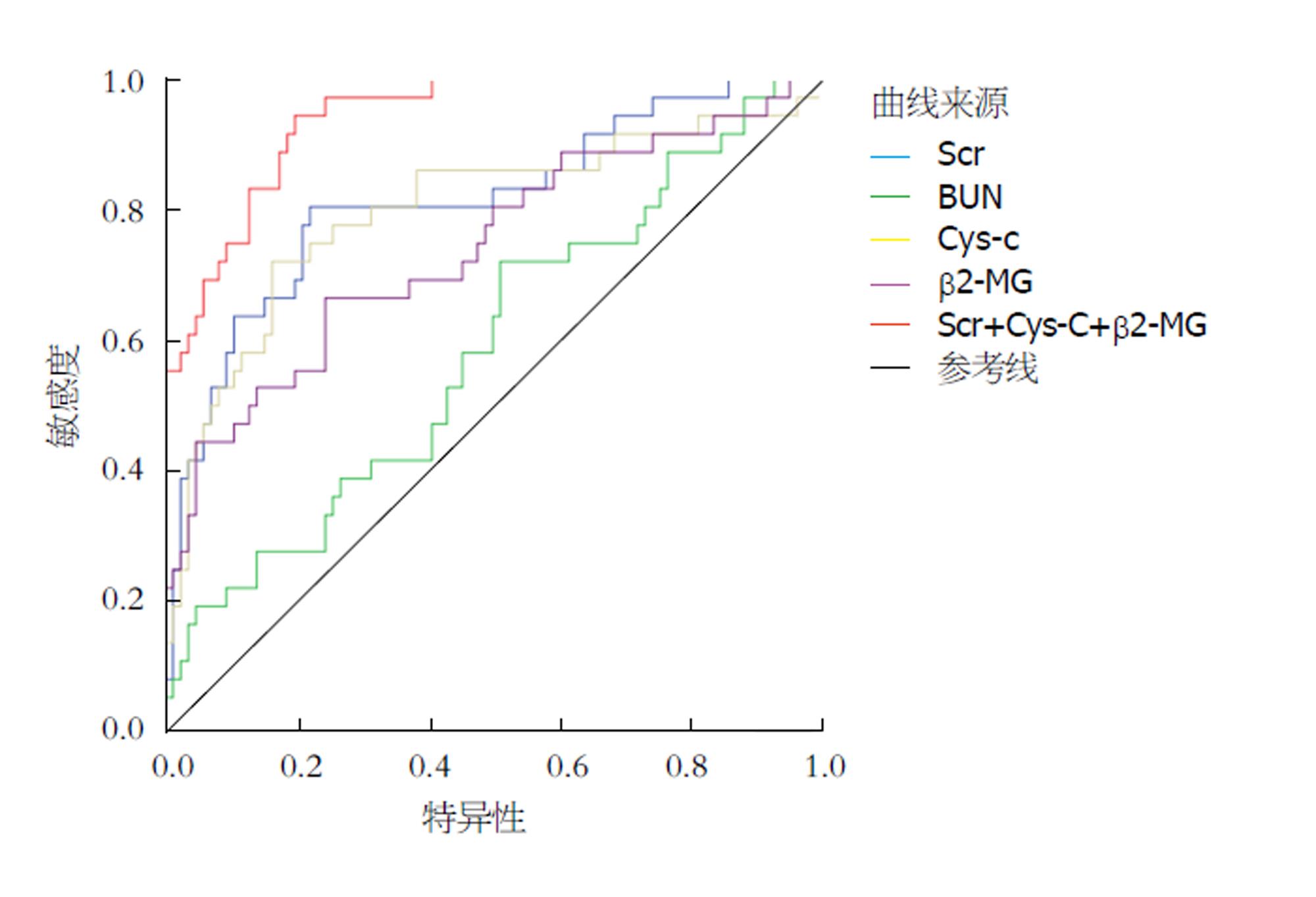
三组患者的Cys-C、β2-MG、Scr和BUN水平等指标间存在统计学差异(F = 47.330、23.693、41.220、26.715; 均P = 0.000); 与CLD和ACLF组相比, HRS患者的Cys-C(t = 9.386、4.807, P = 0.000、0.000)、β2-MG(t = 30.265、4.116, P = 0.000、0.000)、Scr(t = 7.457、7.415, P = 0.000、0.000)和BUN(t = 6.608、5.014, P = 0.000, 0.000)水平均显著升高. ROC曲线显示, 应用4种指标单独预测HRS时, Scr的AUC(0.799)和Cys-C(AUC = 0.789)较高, β2-MG(AUC = 0.741)次之, BUN(AUC = 0.587)最低; 应用Cys-C、β2-MG、Scr联合诊断后的诊断效能(AUC = 0.910)明显高于单独诊断. 以ROC曲线的最佳截点作为预测指标, 3种指标联合预测HRS的诊断准确率80.33%, 灵敏度91.67%, 特异度75.58%, 阳性预测值61.11%, 阴性预测值95.59%, 联合预测的灵敏度显著高于单独诊断(χ2 = 10、8.692、7.432、3.956; P = 0.002、0.003、0.006、0.047).
慢加急性肝衰竭合并肝肾综合征患者Cys-C、β2-MG、Scr和BUN水平显著升高; 应用Cys-C、β2-MG和Scr等3项指标联合预测慢加急性肝衰竭合并肝肾综合征的敏感度较高.
核心提要: 通过对比不同患者胱抑素C(cystatin c, Cys-C)、β2微球蛋白((β2-microglobulin, β2-MG)、血肌酐(serum creatinine, Scr)和血尿素氮(blood urea nitrogen, BUN)水平间的差异, 应用ROC曲线探究出应用Cys-C、β2-MG和Scr等3项指标联合预测慢加急性肝衰竭患者合并肝肾综合征的最佳截点数据, 对于早期诊断患者发生肝肾综合征具有较高的敏感度.
引文著录: 徐晓琳. Cys-C、β2-MG、Scr和BUN水平在预测慢加急性肝衰竭患者合并肝肾综合征中的价值. 世界华人消化杂志 2018; 26(12): 700-706
Revised: March 28, 2018
Accepted: April 4, 2018
Published online: April 28, 2018
To assess the predictive value of cystatin C (Cys-C), β2 macroglobulin (β2-MG), serum creatinine (Scr), and blood urea nitrogen (BUN) for hepatorenal syndrome (HRS) in patients with acute-on-chronic liver failure (ACLF) .
Thirty-six ACLF patients with HRS (HRS group) treated at our hospital from February 2014 to December 2017 were analyzed retrospectively. Thirty-six patients with ACLF without HRS were selected as an ACLF group, and 50 patients with chronic liver disease (CLD) were selected as a CLD group. Cys-C, β2-MG, Scr, and BUN were compared between the three groups. The receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic efficacy of Cys-C, β2-MG, Scr, and BUN, alone or in combination, in predicting HRS in patients with ACLF.
The levels of Cys-C, β2-MG, Scr, and BUN in the three groups were statistically different (F = 47.330, 23.693, 41.220, 26.715; P = 0.000 for all). Compared with the CLD and ACLF groups, Cys-C (t = 9.386, 4.807, P = 0.000 for both), β2-MG (t = 30.265, 4.116; P = 0.000 for both), Scr (t = 7.457, 7.415; P = 0.000 for both), and BUN (t = 6.608, 5.014; P = 0.000 for both) were significantly increased in the HRS group. ROC curve analysis showed that Scr had the highest AUC (0.799), followed by Cys-C (AUC = 0.789), β2-MG (AUC = 0.741), and BUN (AUC = 0.910). The combination of Cys-C, β2-MG, and Scr (AUC = 0.910) performed significantly better than any of the four indexes alone. Using the best cutoff point of the ROC curve as the predictive index, the diagnostic accuracy rate of the combination of Cys-C, β2-MG, and Scr for HRS was 80.33% (sensitivity, 91.67%; specificity, 75.58%; positive predictive value, 61.11%; negative predictive value, 95.59%). The sensitivity of combined indexes was significantly higher than any of the four indexes alone (χ2 = 10, 8.692, 7.432, 3.956; P = 0.002, 0.003, 0.006, 0.047).
The levels of Cys-C, β2-MG, Scr, and BUN in ACLF patients with HRS significantly increase. The combination of Cys-C, β2-MG, and Scr has higher accuracy for predicting HRS in ACLF patients.
- Citation: Xu XL. Value of cystatin C, β2 macroglobulin, serum creatinine, and blood urea nitrogen in predicting hepatorenal syndrome in patients with acute-on-chronic liver failure. Shijie Huaren Xiaohua Zazhi 2018; 26(12): 700-706
- URL: https://www.wjgnet.com/1009-3079/full/v26/i12/700.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v26.i12.700
慢加急性肝衰竭(acute-on-chronic liver failure, ACLF)是指慢性肝病患者在短时间内(一般为4 wk)出现一系列肝功能失代偿症状的症候群[1], 主要的诱发因素有感染(如自发性腹膜炎)、乙型肝炎病毒(hepatitis B virus, HBV)再激活、合并其他嗜肝或非嗜肝病毒感染、大量饮酒、应用肝毒性药物以及手术治疗等[2]. 肝肾综合征是ACLF患者常见的并发症(发病率约为40%), 主要表现为少尿/无尿, 稀释性低钠血症和氮质血症[3], 具有较高的近期死亡率[4]; 由于肝肾综合征(hepatorenal syndrome, HRS)早期表现并不十分特异, 对于ACLF患者早期诊断HRS尚存在一定难度[5]. 血肌酐(serum creatinine, Scr)和血尿素氮(blood urea nitrogen, BUN)是临床常用的反应肾功能的指标, 但对于HRS的敏感度较低; 胱抑素C(cystatin C, Cys-C)与β2微球蛋白(β2 microglobulin, β2-MG)与肾功能早期损伤存在一定相关性, 但在HRS早期诊断中的价值较低[6]. 近来, 有研究报道了应用多种指标联合用于早期诊断HRS, 但具体截点和诊断效能尚不明确. 为此, 我们对绍兴市中心医院36例ACLF合并HRS患者进行回顾性分析, 报告如下.
对2014-02/2017-12于绍兴市中心医院就诊的36例ACLF合并HRS患者(HRS组)进行回顾性分析, 纳入标准: (1)符合《肝衰竭诊治指南(2012年版)》[7]中的慢加急性肝衰竭的诊断标准; (2)肾小球滤过率显著降低: Scr>132.6 mmol/L或24 h肌酐清除率<40 mL/min; (3)停用利尿剂并应用等渗盐水扩容后肾功能无持续改善. 排除标准: (1)合并慢性肾病或近期应用肾毒性药物; (2)合并休克、持续细菌感染和各种原因引起的体液大量丢失; (3)24 h尿蛋白定量<500 mg, 尿常规未见镜下血尿, 泌尿系超声检查无尿路梗阻或肾实质病变. 并选择同时间段内36例ACLF患者(ACLF组)和50例慢性肝病患者(CLD组)作为对照组, 3组患者的一般资料间不存在统计学差异(表1).
| 一般资料 | CLD组 (n = 50) | ACLF组 (n = 36) | HRS组 (n = 36) | F/χ2值 | P值 |
| 年龄(岁) | 43.62 ± 6.29 | 44.55 ± 6.13 | 45.03 ± 6.72 | 0.548 | 0.579 |
| BMI (kg/m2) | 23.54 ± 1.89 | 23.24 ± 1.90 | 23.04 ± 1.46 | 0.861 | 0.425 |
| 性别 n (%) | |||||
| 男 | 28 (56.00) | 20 (55.56) | 23 (63.89) | 0.682 | 0.771 |
| 女 | 22 (44.00) | 16 (44.44) | 13 (36.11) | ||
| 肝病类型 n (%) | |||||
| 乙型肝炎 | 46 (92.00) | 34 (94.44) | 32 (88.89) | 0.743 | 0.691 |
| 丙型肝炎 | 4 (8.00) | 2 (5.56) | 4 (11.11) | ||
| 肝病病史 (年) | 7.08 ± 3.64 | 7.32 ± 3.25 | 8.29 ± 3.66 | 1.298 | 0.277 |
| 甲胎蛋白 (μg/L) | 41.73 ± 7.18 | 42.73 ± 7.19 | 45.59 ± 8.86 | 2.701 | 0.071 |
| PTA (%) | 37.01 ± 17.31 | 33.65 ± 15.27 | 29.36 ± 9.31 | 2.818 | 0.064 |
| TBil (μmol/L) | 169.83 ± 38.89 | 178.95 ± 37.77 | 190.95 ± 39.51 | 2.908 | 0.058 |
1.2.1 检查方案: 于清晨患者空腹状态下抽取患者静脉血样并进行离心, 血浆样本中的Cys-C、β2-MG、Scr和BUN水平应用AU2700全自动生化分析仪(美国贝克曼库尔特)及其配套试剂测定.
1.2.2 评价指标: 比较3组的Cys-C、β2-MG、Scr和BUN水平等临床资料间差异. 以3名副主任医师根据上文诊断做出的临床诊断作为ACLF合并HRS的"金标准", 应用受试者工作曲线(receiver operating characteristic curves, ROC曲线)评价应用Cys-C、β2-MG、Scr和BUN水平预测ACLF合并HRS的价值, 并计算4种指标单独和联合预测的诊断效能(包括诊断准确率、敏感性、特异性、阳性预测值和阴性预测值等). 准确率 = 诊断符合人数/总例数×100%, 敏感性 = 真阳性人数/(真阳性人数+假阴性人数)×100%, 特异性 = 真阴性人数/(真阴性人数+假阳性人数)×100%, 阳性预测值 = 真阳性人数/(真阳性人数+假阳性人数)×100%, 阴性预测值 = 真阴性人数/(真阴性人数+假阴性人数)×100%.
统计学处理 采用软件SPSS23.0进行数据处理, 计量资料以mean±SD的形式表示, 组间比较采用方差分析(analysis of variance, ANOVA), 组内两两比较采用S-N-K法; 计数资料采用n(%)的形式表示, χ2检验比较组间差异. 采用ROC曲线评价预测效能, 绘制ROC曲线图, 并计算曲线下面积(area under curve, AUC)及其95%置信区间、标准误和P值, 并寻找最佳截点. 所有检验均为双侧假设检验, 检验水准α = 0.05. 当P<0.05时认为差异具有统计学意义.
3组患者的Cys-C、β2-MG、Scr和BUN水平等指标间存在统计学差异(F = 47.330、23.693、41.220、26.715; 均P = 0.000); 与CLD和ACLF组相比, HRS患者的Cys-C(t = 9.386、4.807, P = 0.000、0.000)、β2-MG(t = 30.265、4.116, P = 0.000、0.000)、Scr(t = 7.457、7.415, P = 0.000、0.000)和BUN(t = 6.608、5.014, P = 0.000、0.000)水平均显著升高(表2).
ROC曲线显示, 应用4种指标单独预测HRS时, Scr的AUC(0.799)和Cys-C(AUC = 0.789)较高, β2-MG(AUC = 0.741)次之, BUN(AUC = 0.587)最低; 应用Cys-C、β2-MG、Scr联合诊断后的诊断效能(AUC = 0.910)明显高于单独诊断(图1, 表3).
| 检验结果变量 | 截点 | AUC | 标准误差 | P值 | 95%CI | |
| 下限 | 上限 | |||||
| Scr | 114.23 μmol/L | 0.813 | 0.046 | 0.000 | 0.724 | 0.903 |
| BUN | 6.60 mmol/L | 0.587 | 0.057 | 0.031 | 0.475 | 0.698 |
| Cys | 1.31 mg/L | 0.799 | 0.050 | 0.000 | 0.701 | 0.896 |
| β2-MG | 0.27 mg/L | 0.741 | 0.053 | 0.000 | 0.638 | 0.844 |
| 联合诊断 | 0.940 | 0.020 | 0.000 | 0.901 | 0.979 | |
以ROC曲线的最佳截点作为预测指标, 4种指标联合预测HRS的诊断准确率80.33%, 灵敏度91.67%, 特异度75.58%, 阳性预测值61.11%, 阴性预测值95.59%, 联合预测的灵敏度显著高于单独诊断(χ2 = 10、8.692、7.432、3.956; P = 0.002、0.003、0.006、0.047, 表4).
我国是一个肝病大国, 近期调查显示, 我国15-29岁人群乙型肝炎表面抗原阳性率接近5%[8], 据估计全国HBV感染者超过9000万, 其中有2000万慢性乙型肝炎(chronic hepatitis B, CHB)患者[9]. ACLF是CHB患者病情发展的必然趋势和首要死因, 而随着抗病毒治疗的广泛应用, 急性肝衰竭和亚急性肝衰竭的发病率明显降低, ACLF的发生率呈明显升高趋势[10]. HRS是ACLF患者常见的并发症, 其发生机制尚不十分明确, 但肝脏对血管舒张因子的灭活减少所引起的血流动力学异常时其重要原因[11]. 尽管HRS是一种肾脏功能性改变而非器质性改变[12], 但HRS缺乏有效的特异性治疗[13], 患者的近期死亡风险极高[14].
早期预测和诊断HRS的发生, 能够通过采取早期控制和预防感染、纠正贫血、避免肾毒性药物以及维持血容量平衡等措施降低HRS的发生率[15]和严重程度[16]. 本研究通过对比CLD、ACLF和HRS3组不同类型患者的Scr、Cys-C、β2-MG和BUN的差异, 结果显示, 3组患者的Cys-C、β2-MG、Scr和BUN水平等指标间存在统计学差异(P<0.05); 与CLD和ACLF组相比, HRS患者的Cys-C、β2-MG、Scr和BUN水平均显著升高(P<0.05). Scr和BUN是临床常用的反应肾功能的指标, 其中Scr也是HRS诊断的重要指标之一[17], 但由于肾脏具有较为强大的代偿能力以及水钠储溜造成的循环容量升高, 造成Scr对于HRS早期阶段的敏感度较低[18]; BUN的水平则与体内蛋白代谢存在较大相关性, 而ACLF患者的蛋白代谢水平较低, 其在HRS诊断的中的特异性也较差[19]. β2-MG是一种小分子蛋白, 其代谢主要经过肾脏(经肾小球滤过后, 绝大部分经肾小管重吸收, 并由小管上皮细胞分解[20]), 能够反映肾小球和肾小管的滤过和重吸收功能[21], 在多种肾脏疾病的早期阶段中具有重要意义[22]. Cys-C是一种小分子蛋白质, 对半胱氨酸蛋白酶具有抑制作用, 广泛存在于全身组织和体液[23]. Cys-C的产生速率较为恒定, 而且只通过肾小球滤过, 并在近曲小管初被重吸收后, 由小管上皮细胞完全分解[24], 因此被用作反映肾小球滤过率(glomerular filtration rate, GFR)的重要内源性血清标志物[25]. 大量研究显示, 血清Cys-C的水平与GFR具有较高的线性相关性, 其反映GFR的准确性与Scr类似, 甚至在HRS和慢性肾病中晚期优于Scr[26-28].
本研究应ROC曲线比较4种指标预测HRS的效能, 结果显示, 4种指标单独预测HRS时, Scr和Cys-C的AUC较高, β2-MG次之, BUN最低, 这与Ariza等[29]对Cys-C、β2-MG、Scr和BUN等在HRS中的水平变化研究的结论一致. 尽管Scr和Cys-C的AUC较高, 但均未达到0.9, 本研究通过应用Cys-C、β2-MG、Scr等3种指标联合诊断后, 诊断效能得到了明显提高, 明显高于任一单独诊断. 而以ROC曲线的最佳截点作为预测指标, 3种指标联合预测HRS的诊断准确率80.33%, 灵敏度91.67%, 特异度75.58%, 阳性预测值61.11%, 阴性预测值95.59%, 联合预测的灵敏度和阴性预测值显著高于单独诊断, 这提示联合诊断有助于提升对HRS的筛选, 降低漏诊率, 该结论与Mindikoglu等[30]对多项生物标志物与GFR、肾血流量以及肾动脉阻力指数等的相关性和预测价值将为相似.
总之, 慢加急性肝衰竭合并肝肾综合征患者Cys-C、β2-MG、Scr和BUN水平显著升高; 应用Cys-C、β2-MG和Scr等3项指标联合预测慢加急性肝衰竭合并肝肾综合征的敏感度较高.
慢加急性肝衰竭是慢性乙型肝炎(chronic hepatitis B, CHB)患者病情发展的必然趋势和首要死因, 其中, 肝肾综合征是ACLF患者常见的并发症, 早期发现困难, 缺乏有效的治疗手段.
早期预测和诊断慢加急性肝衰竭患者发生肝肾综合征的风险, 能够通过采取早期控制和预防感染、纠正贫血、避免肾毒性药物以及维持血容量平衡等措施, 有效降低HRS的发生率和严重程度; 而肾脏相关血清标志物(Cys-C、β2-MG、Scr和BUN等)被认为在其中具有较好的应用前景.
分析发生和未发生肝肾综合征的慢加急性肝衰竭患者Cys-C、β2-MG、Scr和BUN水平间的差异, 探究上述指标是否与肝肾综合征的发生存在相关性, 并寻找较好的预测指标及其最佳截点数据, 对于早期诊断患者发生肝肾综合征具有较高的敏感度.
通过对比发生和未发生肝肾综合征的慢加急性肝衰竭患者Cys-C、β2-MG、Scr和BUN水平间的差异, 应用回归分析评价上述指标与肝肾综合征的相关性, 并应用ROC曲线对上述指标的诊断价值进行比较, 计算最佳截点数据和诊断效能.
慢加急性肝衰竭合并肝肾综合征患者Cys-C、β2-MG、Scr和BUN水平显著升高; 应用Cys-C、β2-MG和Scr等3项指标联合预测慢加急性肝衰竭合并肝肾综合征的敏感度较高.
肾脏功能相关血清学指标的水平与慢加急性肝衰竭患者发生肝肾综合征的风险有关, 其中, 血清Cys-C、β2-MG和Scr水平与慢加急性肝衰竭患者发生肝肾综合征高度相关. 应用Cys-C、β2-MG和Scr等3项指标联合预测慢加急性肝衰竭合并肝肾综合征的敏感度较高, 有效地提高了对于慢加急性肝衰竭患者发生肝肾综合征的早期预测能力, 为临床早期诊断和治疗慢加急性肝衰竭合并肝肾综合征提供了新的方向.
通过对现有血清学指标进行归纳分析, 或寻找新的肾损伤标志物, 通过大样本、多中心对照研究探究指标与慢加急性肝衰竭患者发生肝肾综合征的相关性, 寻找最佳的诊断模式和截点数据, 进一步提高对于慢加急性肝衰竭合并肝肾综合征的早期预测能力.
学科分类: 胃肠病学和肝病学
手稿来源地: 浙江省
同行评议报告分类
A级 (优秀): 0
B级 (非常好): 0
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
编辑: 闫晋利 电编:张砚梁
| 1. | Durand F, Nadim MK. Management of Acute-on-Chronic Liver Failure. Semin Liver Dis. 2016;36:141-152. [PubMed] [DOI] |
| 2. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [PubMed] [DOI] |
| 3. | Wang X, Sarin SK, Ning Q. Definition of ACLF and inclusion criteria for extra-hepatic organ failure. Hepatol Int. 2015;9:360-365. [PubMed] [DOI] |
| 4. | Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol. 2015;30:236-243. [PubMed] [DOI] |
| 5. | 熊 号峰, 刘 景院. 肝肾综合征研究进展. 中国肝脏病杂志(电子版). 2017;9:1-6. [DOI] |
| 6. | Yap DY, Seto WK, Fung J, Chok SH, Chan SC, Chan GC, Yuen MF, Chan TM. Serum and urinary biomarkers that predict hepatorenal syndrome in patients with advanced cirrhosis. Dig Liver Dis. 2017;49:202-206. [PubMed] [DOI] |
| 8. | Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:7-10. [PubMed] [DOI] |
| 12. | Jindal A, Bhadoria AS, Maiwall R, Sarin SK. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int. 2016;36:59-67. [PubMed] [DOI] |
| 13. | Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9:293-299. [PubMed] [DOI] |
| 14. | Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448-3452. [PubMed] [DOI] |
| 15. | Gifford FJ, Morling JR, Fallowfield JA. Systematic review with meta-analysis: vasoactive drugs for the treatment of hepatorenal syndrome type 1. Aliment Pharmacol Ther. 2017;45:593-603. [PubMed] [DOI] |
| 16. | Hung TH, Lay CJ, Tseng CW, Tsai CC, Tsai CC. The Effect of Renal Function Impairment on the Mortality of Cirrhotic Patients: A Nationwide Population-Based 3-Year Follow-up Study. PLoS One. 2016;11:e0162987. [PubMed] [DOI] |
| 17. | Wang F, Li J, Xing T, Xie Y, Wang N. Serum renalase is related to catecholamine levels and renal function. Clin Exp Nephrol. 2015;19:92-98. [PubMed] [DOI] |
| 18. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [PubMed] [DOI] |
| 19. | Ariza X, Graupera I, Coll M, Solà E, Barreto R, García E, Moreira R, Elia C, Morales-Ruiz M, Llopis M. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57-65. [PubMed] [DOI] |
| 20. | Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol. 2013;305:F390-F395. [PubMed] [DOI] |
| 21. | Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, Romano A, García E, Pavesi M, Risso A. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616-1622. [PubMed] [DOI] |
| 22. | Bucsics T, Schwabl P, Mandorfer M, Bota S, Sieghart W, Ferlitsch A, Trauner M, Reiberger T, Peck-Radosavljevic M. The trigger matters - outcome of hepatorenal syndrome vs. specifically triggered acute kidney injury in cirrhotic patients with ascites. Liver Int. 2016;36:1649-1656. [PubMed] [DOI] |
| 23. | Xue W, Xie Y, Wang Q, Xu W, Mou S, Ni Z. Diagnostic performance of urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin for acute kidney injury in an obstructive nephropathy patient. Nephrology (Carlton). 2014;19:186-194. [PubMed] [DOI] |
| 24. | Ermetici F, Filopanti M, Verga U, Passeri E, Dito G, Malavazos AE, Mapelli C, Raggi ME, Spada A, Corbetta S. Estimated glomerular filtration rate by serum cystatin C correlates with cardiometabolic parameters in patients with primary hyperparathyroidism. Eur J Endocrinol. 2015;173:441-446. [PubMed] [DOI] |
| 25. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884-930. [PubMed] [DOI] |
| 26. | Markwardt D, Holdt L, Steib C, Benesic A, Bendtsen F, Bernardi M, Moreau R, Teupser D, Wendon J, Nevens F. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology. 2017;66:1232-1241. [PubMed] [DOI] |
| 27. | Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O'Connor MF, Devarajan P. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154-2165. [PubMed] [DOI] |
| 28. | Liu J. Evaluation of serum cystatin C for diagnosis of acute rejection after renal transplantation. Transplant Proc. 2012;44:1250-1253. [PubMed] [DOI] |
| 29. | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodríguez E, Huelin P, Solé C. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. [PubMed] [DOI] |
| 30. | Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, Christenson RH, Magder LS, Hutson WR, Seliger SL, Weir MR. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol. 2014;39:543-552. [PubMed] [DOI] |