修回日期: 2016-03-27
接受日期: 2016-04-05
在线出版日期: 2016-05-08
目的: 探讨黄芩苷对肠黏膜上皮细胞肌醇需要蛋白1α的影响, 观察黄芩苷对肠缺血再灌注损伤(intestinal ischemia reperfusion injury, IIRI)的保护作用.
方法: 8 wk, ♂SD大鼠24只, 180-200 g左右, 按随机数字表法分3组: 假手术组(Sham组), 肠缺血再灌注损伤组(I/R组), 黄芩苷预处理组组(黄芩苷+I/R组), 每组8只. Sham组仅开腹关腹. I/R组给予结扎肠系膜上动脉30 min恢复血流再灌注6 h处死, 建立IIRI模型. 黄芩苷+I/R组造模前30 min给予黄芩苷100 mg/kg腹腔注射预处理. 采用ELISA检测小肠组织肿瘤坏死因子-α(tumor necrosis factor α, TNF-α)及血浆小肠脂肪酸结合蛋白(IFABP)的表达, 评价肠组织炎性因子表达水平及肠通透性改变; TUNEL法检测各组肠黏膜上皮细胞凋亡情况; 免疫组织化学检测各组肠黏膜肌醇需要蛋白1α(inositol requiring protein 1α, IRE1α)、磷酸化IRE1α(p-IRE1α)的表达; Western blot检测各组GRP78蛋白表达变化.
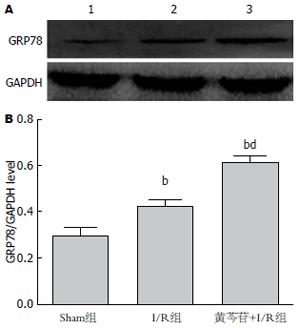
结果: I/R组较Sham组肠黏膜p-IRE1α(41.88±3.43 vs 19.55±2.16)及IRE1α(51.3±4.16 vs 9.97±1.34)、肠组织TNF-α(139.70 ng/L±19.72 ng/L vs 16.41 ng/L±1.75 ng/L)、肠黏膜细胞凋亡指数(40.77%±4.70% vs 3.66%±0.83%)、血浆IFABP(2.25 ng/mL±0.27 ng/mL vs 0.63 ng/mL±0.07 ng/mL)均显著升高(P<0.01). 黄芩苷预处理组较I/R组, GRP78(0.61±0.03 vs 0.42±0.02, P<0.01)表达上调, p-IRE1α(26.71±2.43 vs 41.88±3.43)、IRE1α(36.87±2.07 vs 51.3±4.16)表达降低, 肠组织TNF-α(93.38 ng/L±16.79 ng/L vs 139.70 ng/L±19.72 ng/L)及肠黏膜细胞凋亡水平(29.50%±7.66% vs 40.77%±4.70%)、血浆IFABP浓度(1.50 ng/mL±0.29 ng/mL vs 2.25 ng/mL±0.27 ng/mL)均明显降低(P<0.01).
结论: 黄芩苷可通过上调GRP78缓解内质网应激, 抑制IRE1α过度活化, 减轻IIRI炎性反应及细胞凋亡, 保护肠黏膜屏障.
核心提示: 大鼠肠缺血再灌注损伤(intestinal ischemia reperfusion injury, IIRI)诱发过度内质网应激, 诱导内质网感受蛋白肌醇需要蛋白1α(inositol requiring protein 1α, IRE1α)的上调和过度活化介导肠黏膜上皮细胞炎性反应与细胞凋亡, 黄芩苷通过缓解内质网应激, 抑制IRE1α的过表达及活化保护肠黏膜屏障.
引文著录: 刘希杰, 李智慧, 李丽, 郑步峰, 冯文玉, 程风春, 陈丽君, 傅廷亮. 黄芩苷抑制肌醇需要蛋白1α过度活化减轻肠缺血再灌注损伤. 世界华人消化杂志 2016; 24(13): 1960-1967
Revised: March 27, 2016
Accepted: April 5, 2016
Published online: May 8, 2016
AIM: To investigate the protective effect of baicalin against intestinal ischemia-reperfusion injury (IIRI) and the role of inositol requiring protein 1α (IRE1α) in this process.
METHODS: Twenty-four male Sprague-Dawley rats were randomly divided into three groups (n = 8): a sham group in which rats underwent laparotomy, an IIRI group in which rats were subjected to occlusion of the superior mesenteric artery for 30 min and reperfusion for 6 h, and a baicalin pretreatment group in which rats were given intraperitoneal injection of baicalin (100 mg/kg) about 30 min before IIRI induction. The levels of tumor necrosis factor α (TNF-α) in intestinal tissues and intestinal fatty acid-binding protein (IFABP) in plasma were detected by ELISA. Cell apoptosis was assessed by TUNEL assay. The expression of IRE1α and phosphorylated IRE1α (p-IRE1α) was detected by immunohistochemical staining. Western blot was applied to detect the expression of GRP78 protein in intestinal tissues.
RESULTS: Compared with the sham group, the expression of p-IRE1α (41.88 ± 3.43 vs 19.55 ± 2.16), IRE1α (51.3 ± 4.16 vs 9.97 ± 1.34), the level of TNF-α (139.70 ng/L ± 19.72 ng/L vs 16.41 ng/L ± 1.75 ng/L), cell apoptosis index (40.77% ± 4.70% vs 3.66% ± 0.83%) and IFABP (2.25 ng/mL ± 0.27 ng/mL vs 0.63 ng/mL ± 0.07 ng/mL) were significantly increased in the IIRI group (P < 0.01 for all). Compared with the IIRI group, the expression level of GRP78 (0.60 ± 0.03 vs 0.42 ± 0.02, P < 0.01) was up-regulated, however, the expression of p-IRE1α (26.71 ± 2.43 vs 41.88 ± 3.43) and IRE1α (36.87 ± 2.07 vs 51.39 ± 4.16), the level of TNF-α (93.38 ng/L ± 16.79 ng/L vs 139.70 ng/L ± 19.72 ng/L), cell apoptosis index (29.50% ± 7.66% vs 40.77% ± 4.70%) and IFABP (1.50 ng/mL ± 0.29 ng/mL vs 2.25 ng/mL ± 0.27 ng/mL) were deceased in the baicalin pretreatment group (P < 0.01 for all).
CONCLUSION: Baicalin reduces intestinal ischemia-reperfusion injury by up-regulating GRP78, alleviating endoplasmic reticulum stress and attenuating IRE1α excessive activation.
- Citation: Liu XJ, Li ZH, Li L, Zheng BF, Feng WY, Cheng FC, Chen LJ, Fu TL. Baicalin protects against intestinal ischemia-reperfusion injury by attenuating excessive activation of inositol requiring protein 1α. Shijie Huaren Xiaohua Zazhi 2016; 24(13): 1960-1967
- URL: https://www.wjgnet.com/1009-3079/full/v24/i13/1960.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v24.i13.1960
小肠缺血再灌注损伤(intestinal ischemia reperfusion injury, IIRI)不仅引起肠黏膜屏障受损, 肠道细菌易位、炎性细胞因子大量释放, 还造成远隔器官的损伤[1]; 严重者危及患者生命. 临床上实践中, 维持肠黏膜屏障的完整性对于患者疾病的转归及降低术后感染等并发症具有积极意义[2]. Ca2+超载与氧自由基(reactive oxygen species, ROS)是研究者一直关注的缺血再灌注(ischemia reperfusion, I/R)损伤机制[3]. I/R时内质网Ca2+耗竭、细胞ROS增加, 细胞内未折叠或错误蛋白堆积, 造成内质网负荷增加, 诱发内质网应激(endoplasmic reticulum stress, ERS)[4]. IIRI时, 肠道由于应激增加, 肠黏膜细胞蛋白合成与分泌功能的增加, 使细胞内质网负荷加重. 肌醇需要蛋白1α(inositol requiring protein 1α, IRE1α)作为重要的ERS感受蛋白, 其活化程度决定细胞生存与凋亡[5]. 研究[6]显示, 黄芩苷具有抗氧化、清除自由基, 保护IIRI的作用. 本研究拟通过建立IIRI模型, 同时给予黄芩苷干预, 观察黄芩苷对IIRI的保护作用, 并探讨其对GRP78及IRE1α蛋白的影响.
10%水合氯醛溶液(滨州医学院附属医院制剂室配制); 黄芩苷(南京泽朗医药科技有限公司); 小肠脂肪酸结合蛋白(intestinal fatty acid-binding protein, IFABP)、肿瘤坏死因子-α(tumor necrosis factor α, TNF-α)ELISA检测试剂盒(上海朗顿生物技术有限公司); TUNEL原位凋亡检测试剂盒(南京凯基生物科技发展有限公司); IRE1α(Santa Cruz, 1:300)、p-IRE1α(北京博奥森生物技术有限公司, 1:200)、GRP78(Abcam, 1:5000)、GAPDH(Cell Signaling Technology, 1:1000), 免疫组织化学即用型试剂盒(武汉博士德生物工程有限公司); BCA蛋白定量试剂盒, RIPA裂解液(上海索莱宝生物科技有限公司); 辣根过氧化物酶标记山羊抗兔IgG抗体(北京中杉金桥生物技术有限公司); 电化学发光(ECL)试剂盒.
1.2.1 动物及分组: 8 wk, ♂ SD大鼠24只, 清洁级, 购自山东鲁抗医药股份有限公司[许可证号SCXK(鲁)20130001], 180-200 g体质量, 适应性喂养1 wk后, 将24只大鼠随机分为假手术组(Sham组); 肠缺血再灌注组(I/R组), 黄芩苷预处理组(黄芩苷+I/R组), 每组8只.
1.2.2 模型建立与取材: Sham组仅开腹, 关腹. I/R组于模型建立前禁食1 d, 自由饮水, 10%水合氯醛溶液腹腔内注射(3.5 mL/kg)麻醉后取仰卧位, 备皮, 消毒取腹部正中切口, 长约2 cm, 依次切开入腹, 寻找并游离肠系膜上动脉, 无创血管夹夹闭其根部阻断血流30 min, 松开血管夹, 恢复血流, 造成IIRI模型. 黄芩苷+I/R组于造模前30 min给予黄芩苷100 mg/kg腹腔注射处理. Sham组与I/R组给予等体积PBS溶液腹腔注射, 3组分别于再灌注6 h处死, 取距回盲部6 cm处近端长约2 cm小肠标本, 分两部分, 分别用4%多聚甲醛固定和置于-80 ℃冰箱备用.
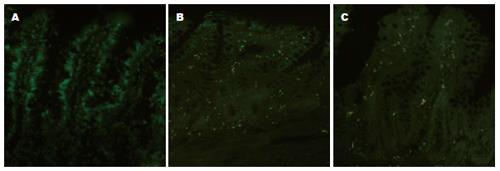
1.2.3 TUNEL原位末端标记法检测肠黏膜细胞凋亡: 按照试剂盒说明操作; 细胞核中有绿色颗粒者为阳性细胞, 计数5个高倍镜×320下的凋亡细胞数, 凋亡指率(apoptotic index, AI) = 检测到的凋亡细胞数/5个高倍视野检测到的细胞总数×l00%.
1.2.4 ELISA检测肠组织TNF-α及血浆IFABP的浓度: ELISA测定肠组织匀浆TNF-α及血浆IFABP浓度. 首先加入标准品和样品后, 加人生物素标记二抗和酶标试剂, 37 ℃反应60 min, 洗板5次, 加入显色剂A和B, 37 ℃显色10 min后加入终止液, 10 min内在562 nm波长下测定吸光度值. 用标准品吸光度建立标准曲线, 计算标本组织TNF-α及血浆IFABP浓度.
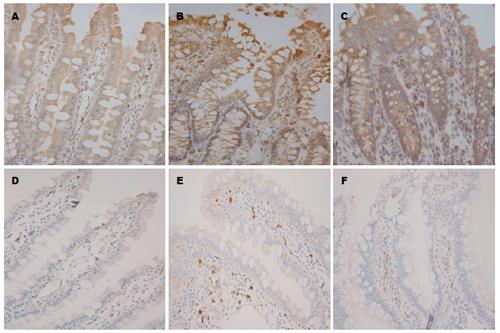
1.2.5 免疫组织化学检测p-IRE1α、IRE1α在肠黏膜组织中的表达: 取4 μm石蜡组织切片, 按免疫组织化学试剂盒说明书分别检测p-IRE1α、IRE1α在各组肠黏膜组织中的表达情况, 并通过Image-Pro Plus Version 6.0图像分析软件检测光密度累计值(integrated option density, IOD).
1.2.6 蛋白免疫印迹法检测肠组织GRP78的表达: 全细胞裂解液提取小肠组织总蛋白, BCA法定量检测蛋白浓度, 置-80 ℃保存. 取50 μg样品常规进行十二烷基磺酸钠-聚丙烯酰胺凝胶电泳后, 电转移至PVDF膜, 用含7%脱脂奶粉的TBST溶液封闭2 h, 加入1:5000稀释的抗GRP78抗体, 4 ℃过夜孵育, 洗膜后加入1:5000稀释的二抗于室温孵育45 min, 最后加入ECL试剂显色. 通过Image J图像分析软件检测其灰度值.
统计学处理 实验数据以mean±SD表示, 采用SPSS16.0统计软件进行单因素方差分析(One-way ANOVA)多组间比较, P<0.05为差异有统计学意义.
较I/R组, 黄芩苷预处理组肠组织TNF-α水平及肠黏膜上皮细胞AI明显降低, 血浆IFABP的含量降低. 提示黄芩苷可明显抑制IIRI肠上皮细胞炎性反应, 减少细胞凋亡, 减轻IIRI, 降低肠通透性(表1, 图1).
较Sham组, I/R组GRP78上调, 提示ERS参与IIRI, 给予黄芩苷预处理组, GRP78表达量较I/R组明显增加, 且差异有统计学意义(P<0.01)(图2). GRP78具有促进未折叠蛋白和错误折叠蛋白的折叠, 缓解ERS的作用, 说明黄芩苷能够上调IIRI过程中GRP78缓解ERS.
免疫组织化学显示, 较Sham组, IIRI时肠黏膜IRE1α表达量增加, 同时活化形式p-IRE1α表达量明显上调, 给予黄芩苷干预后p-IRE1α下调明显, IRE1α表达下调, 说明黄芩苷可抑制IIRI时IRE1α的表达与过度活化(表2, 图3).
IIRI是存在肠扭转、休克、肠移植等多种急危重症疾病的病理生理过程. IIRI所引起的高致死率较前没有明显改善, 因此, 积极探索IIRI的机制和治疗方法对改善IIRI具有重要意义[7]. ROS的大量生成被认为是造成I/R组织损伤的主要因素[8]. I/R损伤造成的中性粒细胞聚集、Ca2+超载及大量ROS的生成可诱导TNF-α和白介素-6(interleukin 6, IL-6)等炎性因子表达水平升高和组织损伤加重[9,10]. 过度炎性反应和肠上皮细胞凋亡导致肠黏膜机械屏障受损, 肠通透性增加[11]. Du等[12]发现1,25(OH)2D3可以通过抑制核因子-κB(nuclear factor-κB, NF-κB)的活化抑制肠上皮细胞肌球轻链蛋白激酶的表达, 促使肌球轻链蛋白磷酸化降低, 降低肠上皮细胞骨架收缩, 缓解TNF-α刺激下的肠黏膜通透性的增加, 保护肠黏膜机械屏障的稳定. Ye等[13]发现TNF-α干预可上调肠上皮细胞及肠组织miR-122a表达, 干扰肠紧密连接蛋白mRNA的转录, 使肠道紧密连接蛋白表达降低, 肠道通透性增加. 给予TNF-α抑制剂可减轻大鼠肠移植后的长期炎性反应[14]. 抗髓过氧化物酶的生成和增加肠组织超氧化物歧化酶的含量, 抑制NF-κB的激活可明显减少肠黏膜TNF-α和IL-6的生成, 减轻肠黏膜过度炎性反应[9]. 本实验显示, I/R组较Sham组相比; 肠组织TNF-α、肠黏膜炎性反应加重. 给予黄芩苷预处理可明显减少肠组织TNF-α的含量.
IIRI引发的早期损伤部位主要黏膜及黏膜下层, 如不及时干预则会累及肠道全层. IIRI后肠黏膜ROS增加及Ca2+失衡是诱发ERS的主要因素, ERS诱导GRP78蛋白上调, 促进未折叠蛋白的折叠和错误折叠蛋白的降解, 降低内质网负荷, 恢复内质网稳态[4], 减少应激细胞凋亡; Lei等[15]通过给予胰高血糖素样肽2干预完全肠外营养小鼠可明显提高肠黏膜抗氧化能力和上调GRP78表达, 减轻肠黏膜上皮细胞的凋亡和萎缩. Bilecová-Rabajdová等[16]研究发现IIRI时随着GRP78表达的上调, 肠上皮细胞凋亡减少, 肠组织损伤减轻. 本实验结果显示, 较I/R组, 黄芩苷预处理后, 肠组织GRP78表达明显升高, 且差异存在统计学意义(P<0.01), 说明黄芩苷能缓解IIRI的ERS.
IRE1α作为ERS主要感受器之一, 广泛表达肠组织细胞. IRE1α的活化能够介导细胞ERS引起的细胞生存和凋亡[17]. 细胞发生ERS时, IRE1α发生磷酸化而活化. 活化的IRE1α可特异性剪切XBP1 mRNA抑制序列, XBP1蛋白表达上调, 促使促折叠蛋白表达上调, 使错误折叠蛋白减少, 减轻内质网负荷. 然而, IRE1α的过度活化其内切酶作用主要是诱导细胞损伤或是凋亡[18]. Lerner等[18]发现持续或过强的ERS, IRE1α的过度活化降解miR17, 使得硫氧蛋白互作蛋白(thioredoxin-interacting protein, TXNIP)的mRNA上调, TXNIP表达增加, 进而上调ERS细胞ROS的含量增加介导细胞损伤和凋亡. 本实验研究显示给予黄芩苷干预后IRE1α的表达较I/R组减少(P<0.01). 另外, 活化的IRE1α可以募集TRAF2, 活化JNK、p38MAPK及NF-κB等炎性信号转导因子, 诱导炎性因子(TNF-α、IL-6)的表达和细胞凋亡的发生[4]. Adolph等[19]研究发现敲除小鼠肠上皮细胞IEC6的XBP1基因, 更容易导致ERS, 诱发肠黏膜细胞炎性反应, 其机制可能与IRE1α活化上调JNK介导过度炎性反应和细胞凋亡有关. 本实验免疫组织化学结果显示, 黄芩苷+I/R组较I/R组, 肠黏膜p-IRE1α的表达量明显降低(P<0.01).
ROS介导ERS激活Caspase12是诱导细胞凋亡的一个重要途径[20]. 核转录因子Nrf2与ERS之间存在紧密联系, Nrf2的抗氧化作用能够缓解ERS诱发的UPR[21]. Nrf2能够上调内质网伴侣基因如HSP90及ERAD的表达, 缓解ERS[22]. 黄芩苷具有抗氧化、抗炎[23], 降低核转录因子NF-κB的活性[24], 减轻炎性反应和细胞凋亡的作用. Lin等[25]发现黄芩苷可以抑制肾I/R损伤过程中TLR4, MyD88和凋亡蛋白Caspase3的活化对肾脏起保护作用. Shen等[26]用黄芩苷检测对衣霉素诱导心肌细胞ERS的影响, 发现黄芩苷可以上调一氧化氮合酶减少细胞凋亡.实验结果显示, 黄芩苷能够使GRP78表达上调, 而IRE1α的表达及活化降低, 炎性因子TNF-α的表达降低, 细胞凋亡减少.
生理条件下, IFABP在血浆和尿液中检测极少, 当肠上皮细胞受损时, IFABP可直接释放入血[27]. 研究[28,29]证实, 血浆IFABP的含量是检测肠黏膜损伤比较精确的指标, 且其敏感性表现为在肠黏膜在轻微损伤时即可测得, 并且IFABP含量的高低与肠黏膜的损伤程度呈现正相关. 研究[30]认为重症患者肠衰竭患者IFABP含量的高低与全身炎症反应综合征及肠道细菌易位有密切联系. 本实验结果提示: 较I/R组, 黄芩苷+I/R组大鼠血浆IFABP含量明显降低, 改善肠黏膜通透性, 保护肠黏膜屏障.
黄芩苷能够保护IIRI, 降低I/R肠上皮细胞的凋亡及过度炎性反应, 保护肠黏膜屏障. 其机制可能是黄芩苷通过抗氧化缓解IIRI过度ERS, 抑制IRE1α过度活化引起的细胞炎症反应和凋亡有关.
肠缺血再灌注损伤(intestinal ischemia reperfusion injury, IIRI)的机制主要是氧自由基(reactive oxygen species, ROS)及Ca2+超载. 研究表明, 肠黏膜肌醇需要蛋白1α(inositol requiring protein 1α, IRE1α)的过度活化可介导ROS的生成, 并且过度内质网应激(endoplasmic reticulum stress, ERS)时IRE1α表达上调和活化主要介导细胞凋亡及炎性反应. 然而, 黄芩苷具有清除氧自由基的作用. 因此, 需要进一步了解黄芩苷对IIRI肠组织IRE1α活化及表达的影响.
向进见, 副主任医师, 长江大学附属第一医院普通外科
关于IIRI的防治主要是针对抗氧化应激, 更好地维护肠黏膜屏障是稳定机体内环境的前提.
Grootjans报道人小肠缺血再灌注损伤过程中Paneth的凋亡情况与未折叠蛋白反应密切相关.
本文研究是基于黄芩苷具抗氧化功能的基础上, 探讨黄芩苷对IRE1α表达的影响.
从ERS角度探讨IIRI的分子机制, 尝试寻找黄芩苷治疗IIRI过程中对ERS相关蛋白分子的调节.
内质网应激: 由于各种原因引起的内质网中出现错误折叠与未折叠蛋白在腔内聚集以及Ca2+平衡紊乱的状态.
IIRI造成的氧化应激及Ca2+超载为ERS的发生创造了条件, 本文从ERS角度探讨IIRI的机制, 对于治疗IIRI有一定的指导意义.
编辑: 于明茜 电编:都珍珍
| 1. | Idrovo JP, Yang WL, Jacob A, Aziz M, Nicastro J, Coppa GF, Wang P. AICAR attenuates organ injury and inflammatory response after intestinal ischemia and reperfusion. Mol Med. 2014;20:676-683. [PubMed] |
| 3. | Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016;23:254-263. [PubMed] [DOI] |
| 4. | Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213. [PubMed] [DOI] |
| 5. | Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562-575. [PubMed] [DOI] |
| 6. | 程 风春, 耿 磊, 陈 强谱, 冯 文玉, 张 兴元, 傅 廷亮. 黄芩苷减轻大鼠肠缺血再灌注损伤及对Nrf2、HO-1表达的影响. 世界华人消化杂志. 2014;22:1510-1517. [DOI] |
| 7. | Grootjans J, Lenaerts K, Buurman WA, Dejong CH, Derikx JP. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J Gastroenterol. 2016;22:2760-2770. [PubMed] [DOI] |
| 8. | Akinrinmade FJ, Akinrinde AS, Soyemi OO, Oyagbemi AA. Antioxidant Potential of the Methanol Extract of Parquetina nigrescens Mediates Protection Against Intestinal Ischemia-Reperfusion Injury in Rats. J Diet Suppl. 2016;13:420-432. [PubMed] [DOI] |
| 9. | Zabot GP, Carvalhal GF, Marroni NP, Hartmann RM, da Silva VD, Fillmann HS. Glutamine prevents oxidative stress in a model of mesenteric ischemia and reperfusion. World J Gastroenterol. 2014;20:11406-11414. [PubMed] [DOI] |
| 10. | Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329-354. [PubMed] [DOI] |
| 11. | Liu XH, Yang YW, Dai HT, Cai SW, Chen RH, Ye ZQ. Protective role of adiponectin in a rat model of intestinal ischemia reperfusion injury. World J Gastroenterol. 2015;21:13250-13258. [PubMed] [DOI] |
| 12. | Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y, Ge X, Nie H, Zheng C, Li YC. 1,25-Dihydroxyvitamin D Protects Intestinal Epithelial Barrier by Regulating the Myosin Light Chain Kinase Signaling Pathway. Inflamm Bowel Dis. 2015;21:2495-2506. [PubMed] [DOI] |
| 13. | Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323-1333. [PubMed] [DOI] |
| 14. | Gerlach UA, Atanasov G, Wallenta L, Polenz D, Reutzel-Selke A, Kloepfel M, Jurisch A, Marksteiner M, Loddenkemper C, Neuhaus P. Short-term TNF-alpha inhibition reduces short-term and long-term inflammatory changes post-ischemia/reperfusion in rat intestinal transplantation. Transplantation. 2014;97:732-739. [PubMed] [DOI] |
| 15. | Lei Q, Bi J, Wang X, Jiang T, Wu C, Tian F, Gao X, Wan X, Zheng H. GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition. Nutrients. 2016;8:pii: E33. [PubMed] [DOI] |
| 16. | Bilecová-Rabajdová M, Urban P, Mašlanková J, Veselá J, Mareková M. Analysis of changes in pro (Gadd153) and anti apoptotic (Grp78) gene expression after ischemic-reperfusion injury of the small intestine. Prague Med Rep. 2010;111:249-256. [PubMed] |
| 17. | Hassler J, Cao SS, Kaufman RJ. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Dev Cell. 2012;23:921-923. [PubMed] [DOI] |
| 18. | Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250-264. [PubMed] [DOI] |
| 19. | Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272-276. [PubMed] [DOI] |
| 20. | Xu X, Liu T, Zhang A, Huo X, Luo Q, Chen Z, Yu L, Li Q, Liu L, Lun ZR. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect Immun. 2012;80:2121-2132. [PubMed] [DOI] |
| 21. | Song G, Zong C, Zhang Z, Yu Y, Yao S, Jiao P, Tian H, Zhai L, Zhao H, Tian S. Molecular hydrogen stabilizes atherosclerotic plaque in low-density lipoprotein receptor-knockout mice. Free Radic Biol Med. 2015;87:58-68. [PubMed] [DOI] |
| 22. | Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89-116. [PubMed] [DOI] |
| 23. | Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, Zhu T. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci. 2014;15:12507-12522. [PubMed] [DOI] |
| 24. | 任 翔, 傅 廷亮, 马 明明, 冯 文玉, 程 风春, 耿 磊, 郑 步峰, 刘 希杰. 黄芩苷对肠上皮细胞缺氧复氧损伤后屏障功能的影响. 中华实用儿科临床杂志. 2015;30:494-497. |
| 25. | Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R, Zhu T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14:19. [PubMed] [DOI] |
| 26. | Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, Zeng C, Xu Y, Shen L, Cheng K. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9:e88389. [PubMed] [DOI] |
| 27. | Khadaroo RG, Fortis S, Salim SY, Streutker C, Churchill TA, Zhang H. I-FABP as biomarker for the early diagnosis of acute mesenteric ischemia and resultant lung injury. PLoS One. 2014;9:e115242. [PubMed] [DOI] |
| 28. | Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, Kitano M. Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg. 2014;101:232-238. [PubMed] [DOI] |
| 29. | Güzel M, Sözüer EM, Salt Ö, İkizceli İ, Akdur O, Yazıcı C. Value of the serum I-FABP level for diagnosing acute mesenteric ischemia. Surg Today. 2014;44:2072-2076. [PubMed] [DOI] |
| 30. | Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22:152-160. [PubMed] [DOI] |