修回日期: 2015-03-28
接受日期: 2015-04-10
在线出版日期: 2015-06-08
目的: 通过观察氧化苦参碱对糖尿病患者空腹血糖、空腹胰岛素、胰岛素抵抗稳态模型指标以及血清中活性氧(reactive oxygen species, ROS)、炎症因子肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)含量的影响, 检测胰岛素抵抗信号通路中关键蛋白的含量变化, 探究氧化苦参碱对胰岛素抵抗的影响及其机制, 为临床上使用氧化苦参碱治疗胰岛素抵抗提供一定的科学依据.
方法: 采用1:1随机对照病例研究方法, 将研究对象分为氧化苦参治疗组和未治疗对照组, 采用葡萄糖氧化酶法检测两组患者空腹血糖、空腹胰岛素水平; 比较两组胰岛素抵抗指数(homeostasis model assessment of insulin resistance, HOMA-IR)和胰岛素敏感指数(homeostasis model assessment of insulin sensitivity index, HOMA-ISI)采用ELISA检测两组患者血清中ROS、TNF-α含量; 采用Western blot检测两组蛋白激酶B(protein kinase B, AKT)、p-AKT、糖原合成酶激酶3α/β(glycogen synthase kinase-3α/β, GSK3α/β)、p-GSK3α/β变化情况.
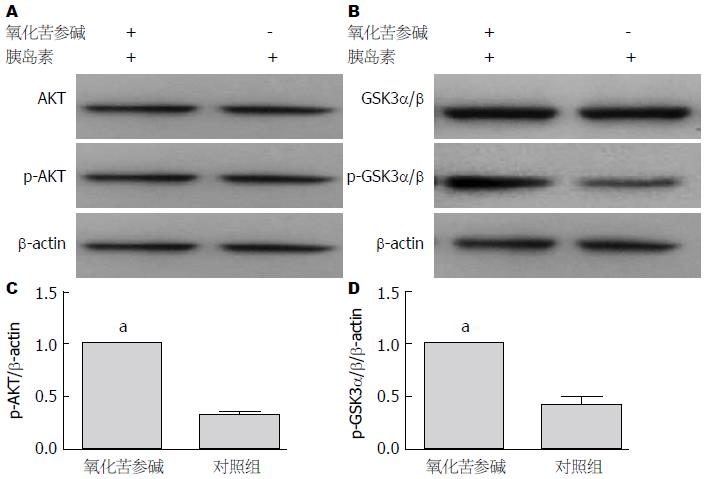
结果: 葡萄糖氧化酶实验结果显示, 经氧化苦参碱治疗后的患者与对照组相比, 空腹血糖、空腹胰岛素显著降低(P<0.05), HOMA-IR降低(P<0.05), HOMA-ISI增加(P<0.05); ELISA结果显示, 经氧化苦参碱治疗后的患者血清中ROS、TNF-α含量显著低于对照组患者(P<0.05); Western blot结果显示, 经氧化苦参碱处理后的肝细胞总蛋白AKT、GSK3α/β含量不变(P>0.05), 而p-AKT、p-GSK3α/β均明显增加(P<0.05).
结论: 氧化苦参碱可降低空腹血糖、空腹胰岛素含量, 一方面通过减少ROS、炎症因子的产生, 改善胰岛素抵抗; 另一方面可以影响胰岛素信号传导通路中关键分子AKT、GSK3α/β的磷酸化, 从而改善胰岛素抵抗, 这也为临床上使用氧化苦参碱治疗胰岛素抵抗提供了基础依据.
核心提示: 氧化苦参碱对糖尿病患者具有改善胰岛素抵抗、降糖、减少机体活性氧产生、减少血清炎症因子等作用, 对临床治疗胰岛素抵抗适应证提供了理论依据.
引文著录: 杜亚萍, 宋光耀, 王富军, 任路平, 刘月芹, 张玉娜, 齐会卿, 丁海霞. 氧化苦参碱对胰岛素抵抗的影响及其作用机制. 世界华人消化杂志 2015; 23(16): 2555-2561
Revised: March 28, 2015
Accepted: April 10, 2015
Published online: June 8, 2015
AIM: To observe the influence of oxymatrine on insulin resistance in patients with type 2 diabetes mellitus and the mechanism involved.
METHODS: This was a prospective randomized controlled clinical study. Patients with type 2 diabetes mellitus were divided into either an oxymatrine treatment group or an untreated group. Glucose oxidase method was used to detect serum fasting blood glucose (FBG) and fasting insulin (FINS). The homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of insulin sensitivity index (HOMA-ISI) were also detected. ELISA was carried out to detect the serum levels of reactive oxygen species (ROS) and tumor necrosis factor-α (TNF-α). Western blot was used to detect the expression of protein kinase B (AKT), p-AKT, glycogen synthase kinase-3α/β (GSK3α/β), and p-GSK3α/β proteins.
RESULTS: FBG, FINS and HOMA-IR significantly decreased and HOMA-ISI increased in the oxymatrine treatment group compared with the untreated group (P < 0.05). Serum levels of ROS and TNF-α in the oxymatrine treatment group decreased significantly compared with the untreated group (P < 0.05). Western blot analysis showed that the total protein levels of AKT and GSK3α/β were unchanged (P > 0.05), but the expression of p-AKT and p-GSK3α/β significantly increased in the oxymatrine treatment group compared with the untreated group (P < 0.05).
CONCLUSION: Oxymatrine can reduce FBG and FINS, and improve insulin resistance by reducing the production of serum ROS and TNF-α and by influencing the photophosphorylation of key proteins (such as AKT and GSK3α/β) in the insulin resistance-related signaling pathways.
- Citation: Du YP, Song GY, Wang FJ, Ren LP, Liu YQ, Zhang YN, Qi HQ, Ding HX. Effect of oxymatrine on insulin resistance in patients with type 2 diabetes mellitus. Shijie Huaren Xiaohua Zazhi 2015; 23(16): 2555-2561
- URL: https://www.wjgnet.com/1009-3079/full/v23/i16/2555.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v23.i16.2555
氧化苦参碱, 又称苦参素, 是从豆科属植物苦参或平科植物广豆根中分离出来的一种生物碱[1], 具有利尿、抗病原体、调血脂、抗氧化、抗炎、抗癌等功效. 有研究[2-5]发现, 氧化苦参碱对肺癌、胃癌细胞诱导的血管内皮细胞增殖具有抑制作用, 也有文献报道[6-8], 氧化苦参碱对糖尿病患者具有改善胰岛素抵抗、降糖等作用. 胰岛素抵抗是指各种原因使胰岛素促进葡萄糖摄取和利用的效率下降, 机体代偿性的分泌过多胰岛素产生高胰岛素血症, 以维持血糖的稳定. 胰岛素抵抗易导致代谢综合征和2型糖尿病. 有研究[9-12]表明, 氧化应激可诱导胰岛素抵抗, 氧化应激是指机体内的活性氧(reactive oxygen species, ROS)生成增加或者清除能力下降导致机体和细胞的损伤, 因此, 在胰岛素抵抗发生过程中, 氧化应激水平增加, 同时, 机体内炎症因子等含量也明显增加.
蛋白激酶B(protein kinase B, AKT)和糖原合成酶激酶3α/β(glycogen synthase kinase-3α/β, GSK3α/β)是胰岛素信号通路中的两个关键蛋白分子, 对于胰岛素信号通路的激活和传导起了重要作用, AKT和GSK3α/β磷酸化的增强提示机体内胰岛素抵抗减弱或有改善, 同时, 机体对胰岛素的敏感度增加[13-15]. 本研究通过观察氧化苦参碱对糖尿病患者空腹血糖(fasting blood glucose, FBG)、空腹胰岛素(fasting insulin, FINS)、胰岛素抵抗稳态模型指标以及血清中ROS、炎症因子肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)含量的影响, 检测胰岛素抵抗信号通路中关键蛋白的含量变化, 为临床上使用氧化苦参碱治疗胰岛素抵抗提供一定的科学依据.
临床研究对象的纳入: (1)随机选取2013-01-01/2014-01-01河北医科大学第四医院内分泌科收治的使用氧化苦参碱治疗的2型糖尿病患者30例(平均年龄36.72岁±3.15岁), 以及2013-01-01/2014-01-01河北医科大学第四医院收治的未使用氧化苦参碱治疗的2型糖尿病患者30例, 作为对照组(平均年龄35.14岁±2.86岁); (2)根据医学论理学原则, 研究前应对研究对象进行告知和讲解, 确保所有研究对象充分了解相关信息, 并签署知情同意书; (3)研究对象无其他病理因素.
1.2.1 标本采集与保存: 实验对象在实验前10 h禁食, 氧化苦参碱治疗组(给予患者口服氧化苦参碱, 3次/d, 每次300 mg), 于治疗第8周时的清晨空腹抽取肘静脉血4 mL, 装在有肝素抗凝的离心管中, 混匀, 离心(5000 r/min, 10 min).
1.2.2 实验室检测: (1)采用日立全自动生化分析仪检测甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)含量: 将患者的血清标本直接加在生物试剂板上, 以标本中的水为溶剂, 使血液中的预测的TG和TC与生物试剂反应面上的固化试剂进行生化反应, 测定其吸光度; (2)采用葡萄糖氧化酶法测定FBG: 在625 nm波长下通过测定反应所生成的蓝色物质的吸光度计算样品中葡萄糖的含量[16]; (3)HOMA评价胰岛β细胞功能以及胰岛素抵抗情况: 胰岛素抵抗指数(homeostasis model assessment of insulin resistance, HOMA-IR) = FINS×FBG/22.5; 胰岛素敏感指数(homeostasis model assessment of insulin sensitivity index, HOMA-ISI) = In[22.5/(FBG×FINS)][17]; (4)采用胰岛素释放实验测定FINS: 首先, 于禁食10 h后的清晨抽取肘静脉血; 其次, 受试者在5 min内饮完含有75 g葡萄糖粉的水溶液250-300 mL, 从喝第一口糖水计时间, 1、2、3 h分别抽血样本; 最后, 采用BACKMAN全自动化学发光免疫分析仪, 通过酶联免疫化学发光法测定FINS; (5)采用ELISA检测血清中ROS、炎症因子TNF-α含量: 将不同浓度的标准品各0.1 mL依次加入一排7孔中, 加入稀释的待检测样品0.1 mL, 置于37 ℃孵育90 min. 将抗体工作液按每孔0.1 mL依次加入, 置于37 ℃孵育60 min, 用PBS洗涤3次; 将ABC工作液按每孔0.1 mL依次加入, 置于37 ℃孵育30 min, 用PBS洗涤5次; 按每孔90 μL依次加入TMB显色液, 置于37 ℃避光反应15-20 min; 加入TMB终止液, 酶标仪在450 nm处测定吸光度[18]; (6)采用Western blot检测人肝细胞株HL7702经氧化苦参碱处理后AKT、p-AKT、GSK3α/β、p-GSK3α/β蛋白含量: 采用碧云天生产的BCA工作液对样品中的蛋白进行定量; 在样品中加入适量碧云天生产的5×的SDS-PAGE蛋白上样缓冲液. 沸水浴中3 min后上样, 电泳(80 V电压30 min, 100 V电压60 min), 电转膜仪转膜(200 mA 90 min), 用5%脱脂牛奶封闭, 随后于摇床上孵育一抗1 h, 4 ℃冰箱过夜, 次日继续孵育一抗1 h, 洗膜3次, 5 min/次, 孵育二抗2 h, 洗膜3次, 10 min/次. 用自动显影仪显影, 并分析条带灰度[19].
统计学处理 采用SPSS19.0对所得结果进行分析, 统计结果采用mean±SD表示. P<0.05为差异有统计学意义.
对临床一般资料的分析可知, 氧化苦参碱治疗组和对照组在使用氧化苦参碱治疗前, 患者在平均年龄、体质量指数(body mass index, BMI)、TG、TC、FBG、FINS含量方面差异无统计学意义(P>0.05)(表1).
| 分组 | BMI(kg/m2) | 平均年龄(岁) | TG(mmol/L) | TC(mmol/L) | FBG(mmol/L) | FINS(mmol/L) |
| 氧化苦参碱组 | 27.78±1.23 | 36.72±3.15 | 6.87±0.52 | 3.97±0.24 | 7.43±0.79 | 14.57±1.38 |
| 对照组 | 26.53±2.24 | 35.14±2.86 | 6.54±0.33 | 4.01±0.35 | 7.21±0.66 | 13.49±2.07 |
| P值 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
给予患者氧化苦参碱治疗8 wk后, 氧化苦参碱治疗组的患者FBG、FINS含量明显低于对照组患者(P<0.05); HOMA-IR明显低于对照组患者(P<0.05); HOMA-ISI明显高于对照组患者(P<0.05)(表2).
| 分组 | HOMA-IR | HOMA-ISI | FBG(mmol/L) | FINS(mmol/L) |
| 氧化苦参碱组 | 2.57±0.87 | -0.14±0.09 | 4.85±0.78 | 7.67±1.53 |
| 对照组 | 4.76±0.52 | -1.39±0.12 | 7.17±0.55 | 13.14±1.69 |
| P值 | <0.05 | <0.05 | <0.05 | <0.05 |
由生化分析仪检测结果可知, 给予患者氧化苦参碱治疗8 wk后, 氧化苦参碱治疗组的患者TG、TC明显低于对照组患者(P<0.05)(表3).
| 分组 | TG(mmol/L) | TC(mmol/L) | ROS(U/mL) | TNF-α(μg/L) |
| 氧化苦参碱组 | 5.12±0.34 | 2.53±0.15 | 392.47±40.38 | 58.46±9.98 |
| 对照组 | 6.44±0.13 | 4.13±0.27 | 480.69±50.24 | 72.34±10.62 |
| P值 | <0.05 | <0.05 | <0.05 | <0.05 |
由ELISA结果可知, 给予患者氧化苦参碱治疗8 wk后, 氧化苦参碱治疗组患者血清中ROS、炎症因子TNF-α的含量均明显低于未给予氧化苦参碱治疗组的患者(P<0.05)(表3).
首先, 我们选用人肝细胞株HL7702进行实验, 实验分为两组, 一组预先用氧化苦参碱(20 mg/mL)孵育48 h, 另一组作为空白对照, 不加氧化苦参碱; 随后两组均加入胰岛素(10 μg/mL)刺激10 min, 收集蛋白. Western blot结果显示, 经氧化苦参碱处理后的HL7702总蛋白AKT、GSK3α/β含量不变(P>0.05), 而p-AKT、p-GSK3α/β均明显增加(P<0.05)(图1).
胰岛素抵抗是指各种原因使胰岛素促进葡萄糖摄取和利用的效率下降, 机体代偿性的分泌过多胰岛素产生高胰岛素血症, 以维持血糖的稳定. 胰岛素抵抗的病因主要有: 遗传性因素、肥胖、长期高血糖、高游离脂肪酸血症、TNF增多、瘦素抵抗和脂联素水平的降低或活性减弱等[20-25]. 胰岛素信号通路中主要的两个蛋白AKT和GSK3α/β在细胞代谢、细胞周期调控、细胞生长凋亡等多种生物学过程中起到了重要作用, AKT和GSK3α/β磷酸化的增强提示机体内胰岛素抵抗减弱或有改善, 同时, 机体对胰岛素的敏感度增加[26-30].
氧化苦参碱是一种由豆科植物苦参的干燥根、植株、果实经乙醇等有机溶剂提取制成的生物碱. 一般为苦参总碱, 其主要成分有苦参碱、槐果碱、氧化槐果碱、槐定碱等多种生物碱, 其中, 以苦参碱、氧化苦参碱含量最高[31]. 已有报道[32-34]证明, 氧化苦参碱具有降血压、抗心律失常、抗病原微生物、平喘祛痰、利尿等作用. 近年来, 有文献报道[35], 氧化苦参碱对糖尿病患者具有改善胰岛素抵抗、降糖等作用. 本研究主要通过观察氧化苦参碱对糖尿病患者FBG、FINS、胰岛素抵抗稳态模型指标以及血清中ROS、炎症因子TNF-α含量的影响, 检测胰岛素抵抗信号通路中关键蛋白的含量变化, 探究氧化苦参碱对胰岛素抵抗的影响及其机制.
研究结果显示, 给予患者氧化苦参碱治疗8 wk后, 氧化苦参碱治疗组的患者FBG、FINS含量明显低于对照组患者(P<0.05); HOMA-IR明显低于对照组患者(P<0.05); HOMA-ISI明显高于对照组患者(P<0.05); TG、TC明显低于对照组患者(P<0.05); 血清中ROS、炎症因子TNF-α的含量均明显低于对照组患者(P<0.05); 经氧化苦参碱处理后的HL7702总蛋白AKT、GSK3α/β含量不变(P>0.05), 而p-AKT、p-GSK3α/β均明显增加(P<0.05).
研究结果证明, 氧化苦参碱对糖尿病患者具有改善胰岛素抵抗、降糖、减少机体ROS产生、减少血清炎症因子等作用, 这也为临床上使用氧化苦参碱治疗胰岛素抵抗提供了一定的科学依据.
氧化苦参碱具有多种生物活性, 如具有利尿、抗病原体、免疫作用, 临床上主要用于治疗慢性乙型肝炎, 肝纤维化. 近年来发现, 氧化苦参碱对糖尿病患者具有改善胰岛素抵抗、降糖等作用, 而用于2型糖尿病治疗的具体机制并不明了.
周力, 主任医师, 贵阳医学院附属医院消化内科
氧化苦参碱能改善胰岛素抵抗, 具有降糖的作用, 这也为临床上使用氧化苦参碱治疗胰岛素抵抗提供了一定的科学依据.
余小虎等研究发现氧化苦参碱联合二甲双胍对非酒精性脂肪肝有显著疗效且用药安全, 其作用机制可能与胰岛素抵抗指数及血清肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)水平的下降有关, 本文发现氧化苦参碱对2型糖尿病患者具有降糖、血清TNF-α水平和胰岛素抵抗的作用.
氧化苦参碱对2型糖尿病患者具有改善胰岛素抵抗、降糖、减少机体活性氧产生、减少血清炎症因子等作用, 这也为临床上使用氧化苦参碱治疗胰岛素抵抗提供了一定的科学依据.
本研究为临床上使用氧化苦参碱治疗胰岛素抵抗提供了一定的科学依据.
胰岛素抵抗: 是指各种原因使胰岛素促进葡萄糖摄取和利用的效率下降, 机体代偿性的分泌过多胰岛素产生高胰岛素血症, 以维持血糖的稳定.
本文对消化肝病科医生在代谢综合征类患者的非酒精性脂肪性肝病的治疗用药选择方面提供了理论基础, 有较高的临床指导价值.
编辑: 韦元涛 电编:闫晋利
| 1. | Guo C, Zhang C, Li L, Wang Z, Xiao W, Yang Z. Hypoglycemic and hypolipidemic effects of oxymatrine in high-fat diet and streptozotocin-induced diabetic rats. Phytomedicine. 2014;21:807-814. [PubMed] [DOI] |
| 2. | Shi L, Shi L, Zhang H, Hu Z, Wang C, Zhang D, Song G. Oxymatrine ameliorates non-alcoholic fatty liver disease in rats through peroxisome proliferator-activated receptor-α activation. Mol Med Rep. 2013;8:439-445. [PubMed] |
| 3. | Zeng XY, Zhou X, Xu J, Chan SM, Xue CL, Molero JC, Ye JM. Screening for the efficacy on lipid accumulation in 3T3-L1 cells is an effective tool for the identification of new anti-diabetic compounds. Biochem Pharmacol. 2012;84:830-837. [PubMed] [DOI] |
| 4. | Zhang P, Li S, Gao Y, Lu W, Huang K, Ye D, Li X, Chu Y. Novel benzothiazinones (BTOs) as allosteric modulator or substrate competitive inhibitor of glycogen synthase kinase 3β (GSK-3β) with cellular activity of promoting glucose uptake. Bioorg Med Chem Lett. 2014;24:5639-5643. [PubMed] [DOI] |
| 5. | Siegel G, Ermilov E, Knes O, Rodríguez M. Combined lowering of low grade systemic inflammation and insulin resistance in metabolic syndrome patients treated with Ginkgo biloba. Atherosclerosis. 2014;237:584-588. [PubMed] [DOI] |
| 6. | Admyre T, Amrot-Fors L, Andersson M, Bauer M, Bjursell M, Drmota T, Hallen S, Hartleib-Geschwindner J, Lindmark B, Liu J. Inhibition of AMP deaminase activity does not improve glucose control in rodent models of insulin resistance or diabetes. Chem Biol. 2014;21:1486-1496. [PubMed] [DOI] |
| 7. | Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Brönneke H, Collienne U, Hampel B, Wunderlich FT, Schmidt-Supprian M. Distinct Roles for JNK and IKK Activation in Agouti-Related Peptide Neurons in the Development of Obesity and Insulin Resistance. Cell Rep. 2014;9:1495-1506. [PubMed] [DOI] |
| 8. | Rawat AK, Korthikunta V, Gautam S, Pal S, Tadigoppula N, Tamrakar AK, Srivastava AK. 4-Hydroxyisoleucine improves insulin resistance by promoting mitochondrial biogenesis and act through AMPK and Akt dependent pathway. Fitoterapia. 2014;99:307-317. [PubMed] [DOI] |
| 9. | Llaneza-Suarez D, Llaneza P, González C, De-La-Fuente P, García-Ochoa C, Garrido P, Castañón V, Pérez-López FR. Assessment of follicular fluid leptin levels and insulin resistance as outcome predictors in women undergoing in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2014;102:1619-1625. [PubMed] [DOI] |
| 10. | Fan H, Liao Y, Tang Q, Chen XY, Zhang LJ, Liu XX, Zhong M. Role of β2-adrenoceptor-β-arrestin2-nuclear factor-κB signal transduction pathway and intervention effects of oxymatrine in ulcerative colitis. Chin J Integr Med. 2012;18:514-521. [PubMed] [DOI] |
| 11. | Dong XQ, Yu WH, Hu YY, Zhang ZY, Huang M. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm Res. 2011;60:533-539. [PubMed] [DOI] |
| 12. | El Assar M, Angulo J, Santos-Ruiz M, Moreno P, Novials A, Villanueva-Peñacarrillo ML, Rodríguez-Mañas L. Differential effect of amylin on endothelial-dependent vasodilation in mesenteric arteries from control and insulin resistant rats. PLoS One. 2015;10:e0120479. [PubMed] [DOI] |
| 13. | Kato K, Takeshita Y, Misu H, Zen Y, Kaneko S, Takamura T. Liver steatosis is associated with insulin resistance in skeletal muscle rather than in the liver in Japanese patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2015;6:158-163. [PubMed] |
| 14. | Cheung KK, Luk AO, So WY, Ma RC, Kong AP, Chow FC, Chan JC. Testosterone level in men with type 2 diabetes mellitus and related metabolic effects: A review of current evidence. J Diabetes Investig. 2015;6:112-123. [PubMed] |
| 15. | Hagen I, Schulte DM, Müller N, Martinsen J, Türk K, Hedderich J, Schreiber S, Laudes M. Soluble receptor for advanced glycation end products as a potential biomarker to predict weight loss and improvement of insulin sensitivity by a very low calorie diet of obese human subjects. Cytokine. 2015;73:265-269. [PubMed] [DOI] |
| 16. | Verdi H, Tulgar Kınık S, Yılmaz Yalçın Y, Muratoğlu Şahin N, Yazıcı AC, Ataç FB. β-3AR W64R Polymorphism and 30-Minute Post-Challenge Plasma Glucose Levels in Obese Children. J Clin Res Pediatr Endocrinol. 2015;7:7-12. [PubMed] |
| 17. | Wu M, Dai G, Yao J, Hoyt S, Wang L, Mu J. Potentiation of Insulin-Mediated Glucose Lowering without Elevated Hypoglycemia Risk by a Small Molecule Insulin Receptor Modulator. PLoS One. 2015;10:e0122012. [PubMed] [DOI] |
| 18. | Brandon AE, Tid-Ang J, Wright LE, Stuart E, Suryana E, Bentley N, Turner N, Cooney GJ, Ruderman NB, Kraegen EW. Overexpression of SIRT1 in Rat Skeletal Muscle Does Not Alter Glucose Induced Insulin Resistance. PLoS One. 2015;10:e0121959. [PubMed] [DOI] |
| 19. | Kayadibi H, Sertoglu E, Uyanik M. Biochemical view on: Precocious markers of cardiovascular risk and vascular damage in apparently healthy women with previous gestational diabetes. Diabetol Metab Syndr. 2015;7:22. [PubMed] [DOI] |
| 20. | Razzoli M, McCallum J, Gurney A, Engeland WC, Bartolomucci A. Chronic stress aggravates glucose intolerance in leptin receptor-deficient (db/db) mice. Genes Nutr. 2015;10:458. [PubMed] [DOI] |
| 21. | Sivasinprasasn S, Sa-Nguanmoo P, Pratchayasakul W, Kumfu S, Chattipakorn SC, Chattipakorn N. Obese-insulin resistance accelerates and aggravates cardiometabolic disorders and cardiac mitochondrial dysfunction in estrogen-deprived female rats. Age (Dordr). 2015;37:9766. [PubMed] [DOI] |
| 22. | Dou L, Wang S, Sui X, Meng X, Shen T, Huang X, Guo J, Fang W, Man Y, Xi J. MiR-301a Mediates the Effect of IL-6 on the AKT/GSK Pathway and Hepatic Glycogenesis by Regulating PTEN Expression. Cell Physiol Biochem. 2015;35:1413-1424. [PubMed] [DOI] |
| 23. | Paneni F, Costantino S, Cosentino F. Role of oxidative stress in endothelial insulin resistance. World J Diabetes. 2015;6:326-332. [PubMed] [DOI] |
| 24. | Hou ZM, Sun Q, Liu YZ, Chen TF, Tang N. Effects of insulin resistance on myometrial growth. Int J Clin Exp Med. 2015;8:1552-1557. [PubMed] |
| 25. | Wang D, Sun L, Song G, Chen S. Effects of intensive insulin therapy upon pancreatic β cell function in patients newly diagnosed with type II diabetes. Int J Clin Exp Med. 2015;8:1391-1395. [PubMed] |
| 26. | DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A. Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One. 2015;10:e0119995. [PubMed] [DOI] |
| 27. | Sharma BR, Kim HJ, Rhyu DY. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J Transl Med. 2015;13:412. [PubMed] [DOI] |
| 28. | Han L, Ji L, Chang J, Wen J, Zhao W, Shi H, Zhou L, Li Y, Hu R, Hu J. Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol Metab Syndr. 2015;7:14. [PubMed] [DOI] |
| 29. | Bilge U, Ünalacak M, Ünlüoglu I, Ipek M, Çeler Ö, Akalin A. Relationship between 1,25-dihydroxy Vitamin D levels and homeostatic model assessment insulin resistance values in obese subjects. Niger J Clin Pract. 2015;18:377-380. [PubMed] [DOI] |
| 30. | Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr. 2015;6:198-205. [PubMed] [DOI] |
| 31. | Shabani P, Naeimi Khaledi H, Beigy M, Emamgholipour S, Parvaz E, Poustchi H, Doosti M. Circulating Level of CTRP1 in Patients with Nonalcoholic Fatty Liver Disease (NAFLD): Is It through Insulin Resistance? PLoS One. 2015;10:e0118650. [PubMed] [DOI] |
| 32. | Esmaillzadeh A, Zakizadeh E, Faghihimani E, Gohari M, Jazayeri S. The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J Res Med Sci. 2015;20:47-53. [PubMed] |
| 33. | Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991-1006. [PubMed] [DOI] |
| 34. | Rodríguez L, Otero P, Panadero MI, Rodrigo S, Álvarez-Millán JJ, Bocos C. Maternal fructose intake induces insulin resistance and oxidative stress in male, but not female, offspring. J Nutr Metab. 2015;2015:158091. [PubMed] [DOI] |
| 35. | Yokoyama K, Yamada T, Mitani H, Yamada S, Pu S, Yamanashi T, Matsumura H, Nakagome K, Kaneko K. Relationship between hypothalamic-pituitary-adrenal axis dysregulation and insulin resistance in elderly patients with depression. Psychiatry Res. 2015;226:494-498. [PubMed] [DOI] |