修回日期: 2014-02-10
接受日期: 2014-02-19
在线出版日期: 2014-03-28
目的: 探讨乙型肝炎病毒(hepatitis B virus, HBV)感染对肝癌(hepatocellular carcinoma, HCC)组织及外周血中膜联蛋白A2(annexin A2, ANXA2)表达的影响.
方法: 以自身配对法收集30例术后肝癌组织、癌旁组织和远癌组织, 以Western blot法和免疫组织化学法分析肝组织ANXA2表达与细胞中分布; 并以生物素标记的HBV DNA探针检测肝组织中HBV DNA, 以实时定量PCR技术分析肝组织ANXA2 mRNA转录与表达水平; 以ELISA法定量肝病患者血ANXA2水平, 分析其临床价值.
结果: 肝组织ANXA2表达定位于癌细胞的胞浆和胞膜, 癌旁组织定位于胞浆; 癌组织显著高于癌旁组织(F = 498.221, P<0.001), 远癌组织则未见明显表达; 癌灶组织中ANXA2基因呈过转录状态, 显著高于癌旁与远癌组织; 在HBV阳性肝癌组织中, ANXA呈中等至强阳性全数表达, 在HBV阴性肝癌组织ANXA呈低阳性表达, 组间差异显著(Z = 4.933, P<0.001). 血ANXA2水平, 肝癌患者显著高于良性肝病各组(P<0.01), 其表达与HBV感染(t = 6.820, P<0.001)、伴肝外转移(t = 3.191, P = 0.002)、门静脉癌栓(t = 2.859, P = 0.005)、中低程度分化和TNM分期间显著相关(P<0.01), 但与患者性别、年龄、肿瘤大小和甲胎蛋白(alpha-fetoprotein)水平间未见明显相关; 血ANXA2与AFP联检, 其诊断阳性率为96.5%.
结论: HBV相关肝癌ANXA2过表达, 有助于HCC诊断和发病机制解析.
核心提示: 肝癌组织乙型肝炎病毒(hepatitis B virus, HBV) DNA阳性癌组织膜联蛋白亚组分(annexin, ANXA)表达显著高于阴性癌组织组;癌组织ANXA2 mRNA水平显著高于癌旁与远癌组织; 肝癌患者血ANXA2表达与HBV感染、肝外转移、门静脉癌栓、中低程度分化和TNM分期间显著相关; 联检ANXA2与甲胎蛋白(alpha-fetoprotein)诊断肝癌阳性率为96.5%. HBV相关肝癌中ANXA2异常表达, 有助于肝癌(hepatocellular carcinoma)诊断和发病机制解析.
引文著录: 陈雯, 张海健, 顾星, 时运, 钱琦, 姚登福, 陆少林. 乙型肝炎病毒相关肝癌组织及外周血膜联蛋白A2异常表达的临床意义. 世界华人消化杂志 2014; 22(9): 1207-1213
Revised: February 10, 2014
Accepted: February 19, 2014
Published online: March 28, 2014
AIM: To investigate the expression of annexin A2 (ANXA2) in hepatic tissue and serum of hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC).
METHODS: ANXA2 expression in HCC, tumor-adjacent precancerous and distal normal tissues was analyzed by real-time polymerase chain reaction (PCR) and Western blot. The cellular localization of ANXA2 in these specimens was detected by immunohistochemistry, and hepatic HBV DNA was detected by in situ hybridization technique. Levels of serum ANXA2 in patients with HCC or benign liver diseases were quantitatively detected by enzyme-linked immunosorbent assay (ELISA).
RESULTS: Hepatic ANXA2 expression was localized in the cytoplasm and membrane of cells in cancerous tissues, and cytoplasm of cells in tumor adjacent cancerous tissues, but was undetectable in distal normal tissues (F = 498.221, P < 0.001). Overexpression of ANXA2 mRNA in cancerous tissues was significantly higher than that in tumor adjacent cancerous or distal normal tissues. ANXA expression was moderate to strong in cancerous tissues with HBV replication, but low in cancerous tissues without HBV replication (Z = 4.933, P < 0.001). Circulating ANXA2 levels in HCC patients were significantly higher than those in any group of other liver diseases (P < 0.01) except the metastatic liver cancer group. Serum levels of ANXA2 in patients with HCC were associated with HBV infection (t = 6.820, P < 0.001), extrahepatic metastasis (t = 3.191, P = 0.002), presence of portal vein thrombus (t = 2.859, P = 0.005), lower differentiation degree and TNM stage (P < 0.01), but not with patient sex, age, size or AFP level. Combined detection of serum ANXA2 and AFP substantially improved the diagnostic efficiency (96.5%).
CONCLUSION: Abnormal expression of ANXA2 in HBV-related HCC is useful for diagnosing HCC and understanding its pathogenesis.
- Citation: Chen W, Zhang HJ, Gu X, Shi Y, Qian Q, Yao DF, Lu SL. Overexpression of annexin A2 in hepatic tissue and serum of patients with HBV-associated hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2014; 22(9): 1207-1213
- URL: https://www.wjgnet.com/1009-3079/full/v22/i9/1207.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i9.1207
肝细胞癌(hepatocellular carcinoma, HCC)防治是全球的医学难题[1], 与乙型肝炎病毒(hepatitis B virus, HBV)和丙型肝炎病毒(hepatitis C virus, HCV)的感染密切相关[2], 肝癌早诊和早治是提高患者生存率的有效措施. 手术切除仍是目前的主要手段[3,4], 但并不适合多数已发生转移的患者, 且术后患者生存率不理想[5]. 膜联蛋白亚组分A2(annexin A2, ANXA2)是膜联蛋白家族成员之一, 为钙离子依赖的磷脂酰结合蛋白, 在细胞生长、信号转导调控、恶性转变和HCC发展过程中发挥重要作用[6,7]. 在我国肝癌患者中HBV感染者占80%以上, 尚未见报道ANXA2在HBV相关HCC中的表达和诊断价值评估, 本文通过定量分析了肝癌患者的癌组织中ANXA2表达, 与HBV DNA复制关系及与胞内分布, 定量分析了不同肝病患者外周血中ANXA2的表达, 与HBV感染的关系及对HBV相关HCC诊断价值.
(1)肝组织标本: 以自身配对法收集南通大学附属医院住院治疗HCC患者术后肝癌、癌旁(离癌灶>3 cm)和远癌组织(离癌灶>5 cm)各30份, 于液氮中保存. 从部分组织中制备总RNA, 以分析癌组织不同部分中ANXA2 mRNA表达; 再将部分组织匀浆后制备蛋白, 以Western blot法分析ANXA2表达水平. HCC诊断依据全国肝癌研究协作组制定的标准核实[8]. 事先经患者知情同意, 并经医院伦理委员会批准; (2)血清样本: 从住院治疗患者中收集肝癌115例(男88例, 女27例), 年龄范围在25-81岁, 平均48.3岁; 其他患者包括慢性肝炎35例、急性肝炎28例、肝硬化38例和转移性肝癌25例(肺癌、胃癌各6例; 急性髓性白血病、乳腺癌各36例; 结肠癌3例; 宫颈癌和前列腺癌各2例); 所有病例经生化检测、病毒性标志物和超声检查确诊, 于清晨采集血液5 mL, 分离血清后备用; 以放射免疫法检测甲胎蛋白(alpha-fetoprotein, AFP); 以30例献血员[肝炎病毒标志物阴性且谷氨酸氨基转移酶(alanine aminotransferase, ALT)正常]血清标本作对照.
1.2.1 总RNA提取和cDNA合成: 用TRIzol试剂(Invitrogen)分离50 mg肝组织总RNA, 琼脂糖凝胶电泳检测总RNA完整性, 核酸蛋白测定仪(Bio-RAD smartspec™ plus)检测其浓度和纯度. 用cDNA合成试剂盒(Fermentas)以1 μg总RNA为模板同等条件下合成ANXA2和β-actin的cDNA.
1.2.2 实时定量PCR: 用stepone™实时定量PCR仪(Applied Biosystems), PCR反应. 50 μL反应体系包含25 μL 2×SYBR Premix Ex Taq(TaKaRa, Japan), 2 μL引物混合物, 1 μL 50×ROX Reference Dye I, 4 μL cDNA, 和18 μL去离子水. ANXA2引物序列: 上游, 5'-TGAGCGGGATGCTTTGAAC-3', 下游5'-ATCCTGTCTCTGTGCATTGCTG-3'; β-actin引物: 上游, 5'-A TTGCCGACAGGATGCAGA-3', 下游, 5'-GAGTACTTGCGCTCAGGAGGA-3'[9], 优化PCR条件如下: 95 ℃ 2 min; 95 ℃ 10 s, 62 ℃ 1 min, 40个循环; 60 ℃ 15 s, 以2-∆∆Ct值相对定量分析.
1.2.3 Western blot分析: 肝组织在预冷匀浆液匀浆(4 ℃), 800 g离心10 min, 收集上清液, 以BCA法(碧云天生物技术有限公司)测定蛋白浓度. 每个样品取20 mg蛋白上样到15%十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)上, 蛋白质然后转移到聚偏氟乙烯膜上, 用5%牛血清白蛋白tris缓冲液4 ℃封闭过夜, 孵育在抗ANXA2和抗β-actin(Santa Cruz)一抗中4 h, 随后在连接有辣根过氧化物酶二抗中孵育1 h, 然后条带在化学发光系统(Millipore, USA)中可视化. 用image J软件分析蛋白密度. 用相对比值(RR)比较ANXA2表达, RR = SIANXA2/SIβ-actin, SI表示蛋白信号强度.
1.2.4 免疫组织化学染色: 采用Elivision一步法进行免疫组织化学. 10%甲醛固定24 h、在梯增稀释的乙醇中脱水(5 min/次), 石蜡包埋, 制成厚度3 μm的组织切片. 用二甲苯脱蜡两次(10 min/次), 在梯减稀释的乙醇中脱水(5 min/次). 脱蜡的切片用pH 7.4的PBS洗涤3次(5 min/次), 孵育在内源性过氧化物酶封闭液中, 0.01 mol/L pH 6.0的柠檬酸盐缓冲液处理10 min, 高压加热法修复抗原, 正常动物血清封闭非特异性结合, 滴加ANXA2抗体, 4 ℃过夜, PBS缓冲液漂洗, 滴加二抗, 室温孵育10 min, PBS漂洗, 滴加链霉素抗生物素蛋白-过氧化酶, 室温孵育10 min, PBS冲洗, 滴加新鲜配制的DAB溶液, 室温显色. 双蒸水洗涤, 苏木素复染, 无水乙醇脱水透明, 封片. Olympus BX50光学显微镜观察、摄像, 以0.01 mol/L PBS(pH 7.5)分别替代一抗、二抗作阴性对照.
1.2.5 酶联免疫吸附测定: 用人ANXA2 ELISA试剂盒(武汉优尔生科技股份有限公司)检测血清ANXA2表达水平, 100 μL血清样本或者标准品被分别添加到96孔板中, 接着添加100 μL检测试剂A并37 ℃孵育1 h, 随后添加100 μL检测试剂B并37 ℃孵育30 min, 然后添加90 μL底物溶液并37 ℃孵育25 min, 最后每孔添加50 μL终止液并读取450 nm处的吸光度.
统计学处理 数据以mean±SD表示, 以t检验比较两样本均数; 以q检验作多样本均数的两两比较, 以受试者工作特征曲线(receiver operating characteristic curve, ROC)评估外周血ANXA2表达水平对肝癌诊断的灵敏性和特异性[10,11], P<0.05为有统计学意义.
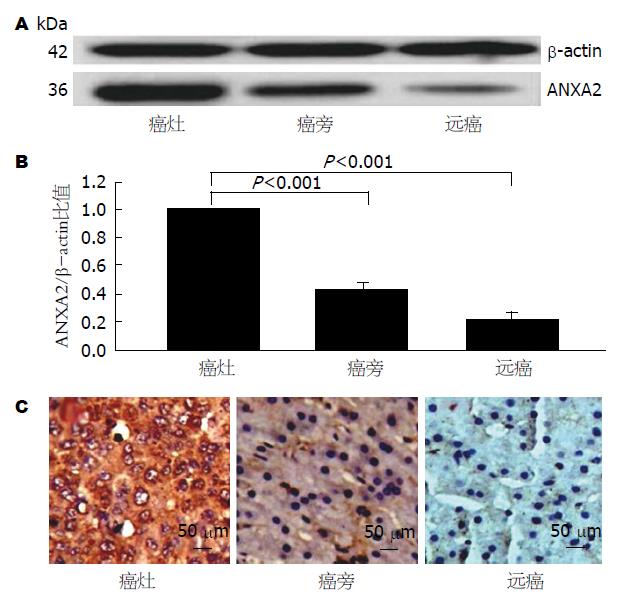
按自身配对法收集肝癌患者术后新鲜的30例肝癌的癌灶、癌旁和远癌组织, 以Western blot法, 相对定量分析肝组织ANXA2蛋白表达(图1A), 经组织ANXA2与β-actin相对定量显示: 癌灶组织ANXA2表达显著高于癌旁组织和远癌组织(F = 498.221, P<0.001, 图1B); 免疫组织化学分析的细胞定位, ANXA2在HCC癌灶组织中定位于胞浆和胞膜, 癌旁中定位于胞浆, 而远癌组织中未见明显表达(图1C).
肝癌不同组织ANXA2 mRNA转录水平如表1. 肝癌的癌灶组织中ANXA2 mRNA相对表达水平(2-∆∆Ct值)明显高于自身配对的癌旁组织或远癌组织, 显示癌灶组织中ANXA2基因呈过转录状态.
肝癌癌灶组织中ANXA全数表达(表2, 100%). 在HBV阳性肝癌组织中, ANXA呈中等至强阳性(++)-(+++)表达, 而在HBV阴性肝癌组织ANXA呈低阳性(+)-(++)表达, 组间差异显著(Z = 4.933, P<0.001).在23例HBV阳性肝癌组中ANXA的表达强度在(+++)以上为22例, 占95.7%: HBV阴性肝癌组中ANXA的表达强度未见强阳性表达(+++), 以中等阳性表达(++)者为6例, 占85.7%.肝癌患者ANXA表达强度, 在HBV阳/阴性组间差异显著.
| HBV DNA | n | 肝细胞中ANXA2表达状态 | Z值 | P值 | |||
| - | + | ++ | +++ | ||||
| 阳性 | 23 | 0 | 0 | 1 | 22 | 4.933 | <0.001 |
| 阴性 | 7 | 0 | 1 | 6 | 0 | ||
肝癌患者血ANXA2表达与HBV感染关系, 在HBsAg阳性组(27.38 ng/mL±5.67 ng/mL)高于阴性组(18.58 ng/mL±7.83 ng/mL), 差异显著(t = 6.820, P<0.001); 伴与不伴肝外转移组分别为26.03 ng/mL±5.99 ng/mL和22.79 ng/mL±5.64 ng/mL, 组间差异显著(t = 3.191, P = 0.002); 伴/不伴有门静脉癌栓组分别为26.03 ng/mL±5.99 ng/mL和23.06 ng/mL±5.03 ng/mL, 差异显著(t = 2.859, P = 0.005); 低分化组为27.05 ng/mL±5.13 ng/mL、中等分化组为26.19 ng/mL±5.34 ng/mL均显著高于高分化组20.43 ng/mL±4.97 ng/mL(P<0.001); TNM分期显示Ⅲ-Ⅳ期为27.44 ng/mL±6.01 ng/mL显著高于Ⅰ-Ⅱ期21.16 ng/mL±5.97 ng/mL(t = 5.594, P<0.001); 但在年龄≥50岁和<50岁组、肿块≥5.0 cm与<5.0 cm组、AFP在≥400 ng/mL组和<400 ng/mL组间, 均未见明显差异.
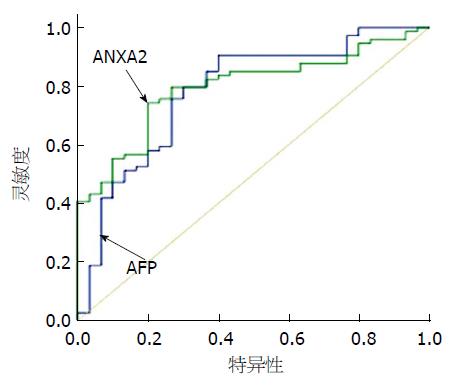
血ANXA2和AFP浓度对HCC诊断价值评估的ROC曲线如图2. 在该曲线下ANXA2的面积为0.903, AFP的面积为0.823. 血ANXA2敏感度是87.0%, 与AFP联合对HCC诊断的灵敏性96.5%, 阴性预测值提高到96.6%.
ANXA2是ANXA家族的重要成员, 其分子的核心岩藻糖基化水平随肿瘤转移潜能增加, 可激活并生成纤溶酶以降解环绕血管的细胞外基质(extracellular matrix, ECM)和基膜, 启动蛋白水解级联反应, 导致肿瘤细胞黏附、浸润和转移, 在肿瘤细胞转移和促血管形成中扮演重要角色[12,13]. 长江口地区为肝癌高发地区, HBV感染是肝癌发生的主要病原学因素[14,15]. ANXA2在肝细胞生长、信号转导和诱发恶性转变中发挥重要作用, 但在HBV相关肝癌患者, HBV感染与ANXA2表达的相互关系还尚未见报道[16,17]. 本文分析了HBV感染相关的肝癌患者, 手术切除标本和患者外周血ANXA2表达水平及其临床价值.
肝组织ANXA2表达显示, 正常肝或慢性肝炎肝组织中, 很少见ANXA2表达. 本研究以自身对照方式分析了肝癌组织的癌灶组织、癌旁组织和远癌组织中ANXA2转录和表达水平[6,10]. 以Western blot法相对定量分析癌灶、癌旁和远癌组织ANXA2表达(图1A)显示: 癌灶组织ANXA2表达显著高于癌旁组织和远癌组织(图1B); ANXA2在肝细胞定位, 免疫组织化学显示ANXA2定位于HCC癌组织的胞浆和胞膜, 癌旁组织胞浆, 远癌组织未见表达(图1C). ANXA2表达与肝细胞的程度分化呈负相关, 低分化肝癌组织中ANXA2表达越高, 高分化肝癌组织ANXA2表达越低. 从转录水平上分析不同肝组织中ANXA2 mRNA表达(表1), 同样发现癌组织中ANXA2表达明显高于自身配对的癌旁或远癌组织. 癌组织中ANXA2过表达, 提示从ANXA2表达参与肝癌的进展过程[6,18], 其水平异常是监测肝细胞恶性转化的有用标志物[19,20].
HBV相关肝癌中AXNA2表达尚未见诸文献[21,22]. 本文资料显示30例肝癌组织中ANXA呈全数阳性表达(表2), 但在HBV阳性肝癌组织中, ANXA2呈中等至强阳性表达占95.7%; 而HBV阴性肝癌组织中, ANXA2表达程度较低, 组间差异显著; 肝癌患者外周血中ANXA2浓度较于正常对照明显升高, 血ANXA2异常有助于肝癌诊断, 且与AFP可互补诊断HCC(图2); ANXA2表达的临床病理学特征显示: 肝癌患者血ANXA2表达与HCC侵袭和转移、HBV感染、分化程度和TNM分期有关, 其机制可能与血纤维蛋白溶酶原/血纤维蛋白溶酶[23]、分子表面的酩氨酸23位磷酸化[24]及核因子(nuclear factor κB, NF-κB)活化等相关[25]. 但与肝癌的瘤体大小度间未见明显相关. 肝癌患者血ANXA2表达与HBV感染呈明显的正相关关系, 在乙肝表面抗原(hepatitis B virus surface antigen, HBsAg)阳性组血ANXA2水平明显高于HBsAg阴性组, 组间差异十分显著, 提示肝组织中HBV复制与ANXA2表达明显相关.
HCC是最常见的恶性肿瘤, 发病率居全球恶性肿瘤的第5位, 死亡率居第3位[26,27]. HCC的发生发展由原癌基因激活和抑癌基因失活、信号传导通路异常、细胞周期调控因子突变、抗凋亡基因激活、血管形成及对放、化疗的耐药性等多种因素共同作用形成, 且发病率仍在持续增加, HCC预后极差, 早期特异诊断与有效治疗极为重要[1,28,29]. 最近证实ANXA2在HCC的诊断中具有较高的灵敏性和特异性, 可作为早期诊断HCC的标志物[10,30]. 对肝组织与外周血ANXA2研究, 显示在HBV感染相关的肝癌进展中, ANXA2过表达, 可为肝细胞恶性转化监测和肝癌的靶向治疗带来新契机, 对此深入研究将有助于揭示HCC发病机制或为HCC的治疗提供新靶点[31,32].
乙型肝炎病毒(hepatitis B virus, HBV)感染是肝癌发生的主要病原学因素. 膜联蛋白亚组分A2(annexin A2, ANXA2)在肝细胞生长、信号转导和诱发恶性转变中发挥重要作用, 但在HBV相关肝癌患者, HBV感染与ANXA2表达的相互关系还尚未见报道. 研究HBV感染相关肝癌组织和患者外周血ANXA2表达水平, 有助于探讨其临床价值.
卢宁, 副主任医师, 兰州军区乌鲁木齐总医院肿瘤科
肝细胞癌防治是全球的医学难题, 与HBV感染密切相关, 早诊及有效治疗是提高患者生存率的有效措施. ANXA2在细胞生长、恶性转变过程中发挥重要作用. 肝癌的手术切除仍是目前的主要手段, 但并不适合多数已发生转移的患者, 且术后患者生存率不理想, 亟待发现其他新方法.
近期Dong和Zhang等报道了肝癌细胞中ANXA2高表达, 以小发夹型RNA特异性地抑制肝癌细胞株中ANXA2 mRNA转录, 可有效抑制肝癌细胞增殖与转移, 是一具有分子靶向作用的治疗方法, 值得深入研究, 具有应用前景与开发价值.
正常肝或慢性肝炎肝组织罕见ANXA2表达. 以自身对照方式分析肝癌、癌旁和远癌组织ANXA2转录和表达. 癌组织ANXA2表达显著异常, 定位于胞浆和胞膜. HBV相关肝癌组织ANXA呈全数阳性表达,呈中等至强阳性表达占95.7%, 提示HBV复制与ANXA2表达明显相关.
ANXA2在肝癌中异常表达和分子中Tyr23残基磷酸化上调. 定量夹心ELISA法检测肝癌患者血清ANXA2表达, 可能有助于肝癌早期诊断及肿瘤浸润和复发监测. 将血ANXA2检查添加到肝癌特异诊断标志物(GPC3、HS-GGT和HSP70)组合中, 可增加诊断的可靠性和客观性, 尤其是HBV相关肝癌诊断, 具有应用前景.
本文内容客观, 实验方法成熟, 观点新颖, 具有一定的科学意义.
编辑: 田滢 电编: 鲁亚静
| 1. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] |
| 2. | Shen YC, Hsu C, Cheng CC, Hu FC, Cheng AL. A critical evaluation of the preventive effect of antiviral therapy on the development of hepatocellular carcinoma in patients with chronic hepatitis C or B: a novel approach by using meta-regression. Oncology. 2012;82:275-289. [PubMed] [DOI] |
| 3. | DuBray BJ, Chapman WC, Anderson CD. Hepatocellular carcinoma: a review of the surgical approaches to management. Mo Med. 2011;108:195-198. [PubMed] |
| 4. | Portolani N, Baiocchi GL, Coniglio A, Tiberio GA, Prestini K, Gheza F, Benetti A, Maria Giulini S. Limited liver resection: a good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn J Clin Oncol. 2011;41:1358-1365. [PubMed] [DOI] |
| 5. | van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47:1789-1797. [PubMed] [DOI] |
| 6. | Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4:199-208. [PubMed] [DOI] |
| 7. | Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood. 2011;118:4789-4797. [PubMed] |
| 8. | Ministry of Health of the People's Republic of China. [Updated standards for the diagnosis and treatment of primary liver cancer]. Zhonghua Ganzangbing Zazhi. 2012;20:419-426. [PubMed] |
| 9. | Zhang HJ, Yao DF, Yao M, Huang H, Wang L, Yan MJ, Yan XD, Gu X, Wu W, Lu SL. Annexin A2 silencing inhibits invasion, migration, and tumorigenic potential of hepatoma cells. World J Gastroenterol. 2013;19:3792-3801. [PubMed] [DOI] |
| 10. | Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, Yan XD, Chen J. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012;18:5897-5904. [PubMed] [DOI] |
| 11. | Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799-806. [PubMed] [DOI] |
| 12. | Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des. 2007;13:3568-3575. [PubMed] [DOI] |
| 13. | O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136-1146. [PubMed] [DOI] |
| 14. | Pan N, Qiu J, Sun H, Miao F, Shi Q, Xu J, Jiang W, Jin H, Xie W, He Y. Combination of human leukocyte antigen and killer cell immunoglobulin-like receptor genetic background influences the onset age of hepatocellular carcinoma in male patients with hepatitis B virus infection. Clin Dev Immunol. 2013;2013:874514. [PubMed] |
| 15. | Pan Y, Zhao L, Chen XM, Gu Y, Shen JG, Liu LM. The XRCC1 Arg399Gln genetic polymorphism contributes to hepatocellular carcinoma susceptibility: an updated meta-analysis. Asian Pac J Cancer Prev. 2013;14:5761-5767. [PubMed] |
| 16. | Liu W, Cao Y, Wang T, Xiang G, Lu J, Zhang J, Hou P. The N-Glycosylation Modification of LHBs (Large Surface Proteins of HBV) Effects on Endoplasmic Reticulum Stress, Cell Proliferation and its Secretion. Hepat Mon. 2013;13:e12280. [PubMed] [DOI] |
| 17. | Li J, Shi W, Gao Y, Yang B, Jing X, Shan S, Wang Y, Du Z. Analysis of microRNA expression profiles in human hepatitis B virus-related hepatocellular carcinoma. Clin Lab. 2013;59:1009-1015. [PubMed] |
| 18. | Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, Shi L, Murota M, Tani J, Kato K. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33:1157-1163. [PubMed] |
| 19. | Longerich T, Haller MT, Mogler C, Aulmann S, Lohmann V, Schirmacher P, Brand K. Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol Res Pract. 2011;207:8-14. [PubMed] [DOI] |
| 20. | Ji NY, Park MY, Kang YH, Lee CI, Kim DG, Yeom YI, Jang YJ, Myung PK, Kim JW, Lee HG. Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int J Mol Med. 2009;24:765-771. [PubMed] |
| 21. | Motavaf M, Safari S, Saffari Jourshari M, Alavian SM. Hepatitis B virus-induced hepatocellular carcinoma: the role of the virus x protein. Acta Virol. 2013;57:389-396. [PubMed] |
| 22. | Fu Y, Wei X, Tang C, Li J, Liu R, Shen A, Wu Z. Circulating microRNA-101 as a potential biomarker for hepatitis B virus-related hepatocellular carcinoma. Oncol Lett. 2013;6:1811-1815. [PubMed] |
| 23. | Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43-66. [PubMed] [DOI] |
| 24. | Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. [PubMed] [DOI] |
| 25. | Dong ZZ, Yao DF, Wu W, Yao M, Yu HB, Shen JJ, Qiu LW, Yao NH, Sai WL, Yang JL. Delayed hepatocarcinogenesis through antiangiogenic intervention in the nuclear factor-kappa B activation pathway in rats. Hepatobiliary Pancreat Dis Int. 2010;9:169-174. [PubMed] |
| 26. | Chen K, Shi W, Xin Z, Wang H, Zhu X, Wu X, Li Z, Li H, Liu Y. Replication of genome wide association studies on hepatocellular carcinoma susceptibility loci in a Chinese population. PLoS One. 2013;8:e77315. [PubMed] [DOI] |
| 27. | Zhang J, Lu C, He S, Wan C, Zhang Y, Cheng C. Decreased expression of Small glutamine-rich tetratricopeptide repeat-containing protein (SGT) correlated with prognosis of Hepatocellular carcinoma. Neoplasma. 2014;61:83-89. [PubMed] [DOI] |
| 28. | Yao M, Wang L, Yan MJ, Gu X, Yan XD, Zhang HJ, Yao DF. Targeting Annexin A2 gene for suppressing invasion and tumor progression of liver cancer. J Pure Appl Microbio. 2013;7:381-388. |
| 29. | Thirion M, Ochiya T. Roles of microRNAs in the hepatitis B virus infection and related diseases. Viruses. 2013;5:2690-2703. [PubMed] [DOI] |
| 30. | Hofmann A, Osman A, Leow CY, Driguez P, McManus DP, Jones MK. Parasite annexins--new molecules with potential for drug and vaccine development. Bioessays. 2010;32:967-976. [PubMed] [DOI] |
| 31. | Dong ZZ, Yao M, Zhang HJ, Wang L, Huang H, Yan MJ, Yao DF. Inhibition of Annexin A2 gene transcription is a promising molecular-targeted for hepatoma cell proliferation and metastasis. Oncology Letters. 2014;7:28-34. |
| 32. | Jiang JH, Gao Q, Shen XZ, Yu Y, Gu FM, Yan J, Pan JF, Jin F, Fan J, Zhou J. An X-chromosomal association study identifies a susceptibility locus at Xq22.1 for hepatitis B virus-related hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2013;37:586-595. [PubMed] [DOI] |