修回日期: 2014-09-03
接受日期: 2014-09-15
在线出版日期: 2014-11-18
目的: 探讨右美托咪定预处理对脓毒血症SD大鼠急性肝脏损伤中肝组织细胞白介素-6(interleukin-6, IL-6)表达及血C-反应蛋白(C-reactive protein, CRP)、降钙素原(procalcitonin, PCT)的影响, 明确其肝脏的保护机制.
方法: 24只♂SD大鼠随机分为3组: 假手术组(sham operation group)(S组)、脓毒血症组(cecum ligation perforation group)(CLP组)及右美托咪定预处理组[dexmedetomidine (Dex) group](CLP+Dex组). 采用经典的盲肠结扎穿孔法建立模型. 术前30 min Dex组腹腔注射右美托咪定100 μg/kg(右美托咪定浓度10 μg/mL); S组和CLP组腹腔注射等容量的0.9% NaCl. 术后48 h心脏采血测定谷草转氨酶(aspartate aminotransferase, AST)、谷丙转氨酶(alanine aminotransferase, ALT)、CRP、PCT的含量; 免疫组织化学法检测肝脏中IL-6蛋白的表达变化, 并作病理形态学检查.
结果: CLP可致肝功能损伤, CLP组与S组相比, AST、ALT、CRP、PCT明显升高, 差异有统计学意义(168.32 U/L±38.36 U/L vs 42.16 U/L±16.52 U/L; 143.65 U/L±38.45 U/L vs 38.64 U/L±24.26 U/L; 81.46 mg/L±26.58 mg/L vs 18.62 mg/L±6.28 mg/L; 2.68 μg/L±1.26 μg/L vs 0.28 μg/L±0.14 μg/L, P<0.01); IL-6蛋白表达升高, 差异有统计学意义(65.87±13.28 vs 15.28±2.87, P<0.01); 显微结构显示较S组加重. 与CLP组相比, CLP+Dex组AST、ALT、CRP、PCT浓度降低, 差异有统计学意义(128.66 U/L±48.38 U/L vs 168.32 U/L±38.36 U/L; 106.32 U/L±46.64 U/L vs 143.65 U/L±38.45 U/L; 55.86 mg/L±18.64 mg/L vs 81.46 mg/L±26.58 mg/L; 1.85 μg/L±0.42 μg/L vs 2.68 μg/L±1.26 μg/L, P<0.05); IL-6表达明显降低(48.24±8.76 vs 65.87±13.28, P<0.05); 显微结构较CLP组减轻.
结论: 右美托咪定预处理能通过降低IL-6蛋白的表达水平, 从而减轻脓毒血症所致的肝脏损伤.
核心提示: 右美托咪啶预处理的脓毒血症SD大鼠可以通过下调白介素-6(interleukin-6)蛋白的表达, 发挥抗炎作用而对脓毒血症所致急性肝脏损伤起到保护作用, 但其保护机制尚局限在动物实验阶段, 因此, 研究脓毒血症后处理措施及有效应用于临床的治疗将是我们医务工作者努力的方向.
引文著录: 周雪玲, 高华, 王丹, 钟超, 钟涛, 秦静廷. 右美托咪定预处理对脓毒血症SD大鼠急性肝脏损伤的影响. 世界华人消化杂志 2014; 22(32): 4943-4947
Revised: September 3, 2014
Accepted: September 15, 2014
Published online: November 18, 2014
AIM: To investigate the effect of pretreatment with dexmedetomidine (Dex) on the expression of interleukin-6 (IL-6) in liver tissue and serum contents of C-reactive protein (CRP) and procalcitonin (PCT) in rats with sepsis-induced liver injury and the underlying mechanism.
METHODS: Twenty-four male SD rats were equally randomized into three groups: a sham operation group (S), a cecum ligation perforation group (CLP) and a Dex group (CLP + Dex). Sepsis-induced liver injury was induced by using classical cecal ligation. Dex (100 μg/kg) was injected intraperitoneally at a concentration of 10 μg/mL 30 min before surgery in the CLP + Dex group, while equal volume of 0.9% NaCl was injected in the S group and CLP group. Rats were killed 48 h after surgery, and blood was collected from the heart to determine serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), CRP, and PCT. Expression of IL-6 in the liver was evaluated by immunohistochemistry. Liver pathological morphology examination was also conducted.
RESULTS: CLP resulted in sepsis induced liver injury, and serum levels of AST, ALT, CRP, and PCT in the CLP group were significantly higher than those in the S group (168.32 U/L ± 38.36 U/L vs 42.16 U/L ± 16.52 U/L; 143.65 U/L ± 38.45 U/L vs 38.64 U/L ± 24.26 U/L; 81.46 mg/L ± 26.58 mg/L vs 18.62 mg/L ± 6.28 mg/L; 2.68 μg/L ± 1.26 μg/L vs 0.28 μg/L ± 0.14 μg/L, P < 0.01). IL-6 expression was enhanced significantly (65.87 ± 13.28 vs 15.28 ± 2.87, P < 0.01) and changes in liver ultrastructure were more severe in the CLP group compared with the S group. Compared with the CLP group, the Dex group had significantly lower serum levels of AST, ALT, CRP and PCT level (128.66 U/L ± 48.38 U/L vs 168.32 U/L ± 38.36 U/L; 106.32 U/L ± 46.64 U/L vs 143.65 U/L ± 38.45 U/L; 55.86 mg/L ± 18.64 mg/L vs 81.46 mg/L ± 26.58 mg/L; 1.85 μg/L ± 0.42 μg/L vs 2.68 μg/L ± 1.26 μg/L, P < 0.05), significantly reduced IL-6 expression (48.24 ± 8.76 vs 65.87 ± 13.28, P < 0.05), and milder ultrastructure changes.
CONCLUSION: Pretreatment with dexmedetomidine can reduce sepsis-induced liver damage possibly via mechanisms associated with regulating IL-6 levels.
- Citation: Zhou XL, Gao H, Wang D, Zhong C, Zhong T, Qin JT. Effect of pretreatment with dexmedetomidine on sepsis induced acute liver injury in rats. Shijie Huaren Xiaohua Zazhi 2014; 22(32): 4943-4947
- URL: https://www.wjgnet.com/1009-3079/full/v22/i32/4943.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i32.4943
脓毒血症是指病原微生物入侵机体后所致的全身性炎症反应综合征(systemic inflammatory response syndrome, SIRS), 可引起多器官功能障碍综合征(multiple organ dysfunction syndrome, MODS), 是临床中面临的难题[1]. 右美托咪啶是一种新型的高选择性α2肾上腺素能受体激动剂, 不仅具有镇静、镇痛、抗焦虑和改善手术期间的心血管稳定性, 还具有对重要脏器如心脏、大脑、肾脏、肝脏及肺等的保护作用[2-5]. 我们观察右美托咪定预处理对脓毒症所致SD大鼠肝脏损伤的影响, 探讨右美托咪定预处理通过降低白介素-6(interleukin-6, IL-6)蛋白的水平而对肝脏产生保护的机制.
健康♂SD大鼠24只, 体质量250 g±30 g, 由桂林医学院实验动物中心提供. 随机分成3组: 假手术组(sham operation group)(S组)、脓毒血症组(cecum ligation perforation group)(CLP组)及右美托咪定预处理组[dexmedetomidine(Dex) group](CLP+Dex组), 每组8只. 所用抗体购自Santa公司.
1.2.1 模型复制及药物处理: 术前实验大鼠术前12 h禁食、禁水, 乙醚吸入麻醉. 取腹部正中切口进入腹腔, 将肠道推向一侧, 找到盲肠. 采用经典的CLP方法复制大鼠脓毒症模型[6]. 游离肠系膜和盲肠, 以4-0丝线环形结扎盲肠根部, 用18号针头在结扎端贯通穿刺一次, 挤出粪便少许, 还纳肠段, 依次缝合腹膜和皮肤. 对照组仅做剖腹、分离盲肠远端、关腹手术. 术后大鼠腹腔注射生理盐水2 mL, 补充术中丢失的液体量. 连续观察24 h, 整个实验过程于22 ℃-25 ℃的环境下进行, 术后让大鼠自由饮水. CLP+Dex组术前30 min Dex组腹腔注射右美托咪定100 μg/kg(右美托咪定浓度10 μg/mL); S组和CLP组腹腔注射等容量的0.9%NaCl.
1.2.2 标本采集: 48 h后处死大鼠. 从大鼠心脏抽血, 离心保存, 由桂林医学院附属医院检验科协助检测谷草转氨酶(aspartate aminotransferase, AST)、谷丙转氨酶(alanine aminotransferase, ALT)、C-反应蛋白(C-reactive protein, CRP)、降钙素原(procalcitonin, PCT)含量. 无菌条件下取部分肝脏组织置40 g/L甲醛溶液固定, 进行免疫组织化学IL-6相关分析及显微镜观察肝脏显微结构改变.
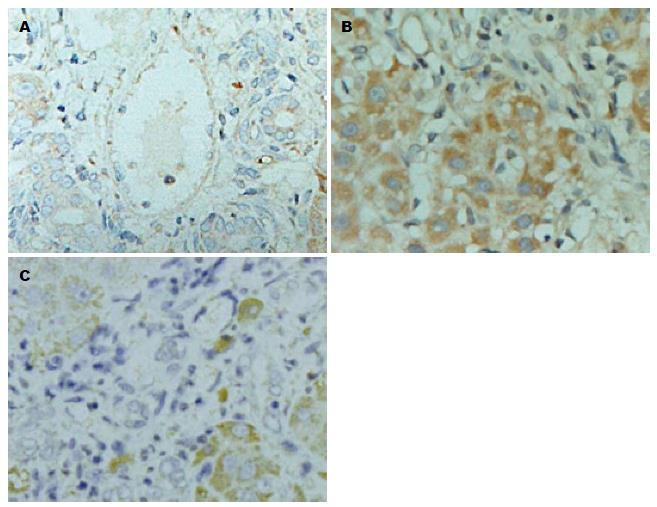
1.2.3 检测指标及方法: (1)肝脏组织切片HE染色及显微结构观察: 全部标本经40 g/L甲醛固定, 梯度脱水, 浸蜡, 包埋, 5 μm连续切片、烤干、脱蜡、HE染色、透明和封片; (2)肝脏IL-6蛋白表达: 制备肝组织切片, 片厚度为3 μm, 免疫组织化学染色采用二步法, 染色步骤按试剂盒说明书进行操作. 细胞核呈棕黄色者为阳性细胞. 采用显微图像分析系统(Olympus AX70/Coolsnapfx加etaMorph)图像处理分析仪检测, 每组在高倍镜下(×400)随机选择6个视野, 测出IL-6蛋白表达的平均吸光度(A)值.
统计学处理 数据采用SPSS13.0统计软件处理, 实验数据以mean±SD表示, 均数用方差分析中的多个样本均数的两两检验. P<0.05被视为差异有统计学意义.
CLP可致肝功能损伤, CLP组与S组相比, AST、ALT、CRP、PCT明显升高, 差异有统计学意义(P<0.05); 与CLP组相比, CLP+Dex组AST、ALT、CRP、PCT浓度降低, 差异有统计学意义(P<0.05)(表1).
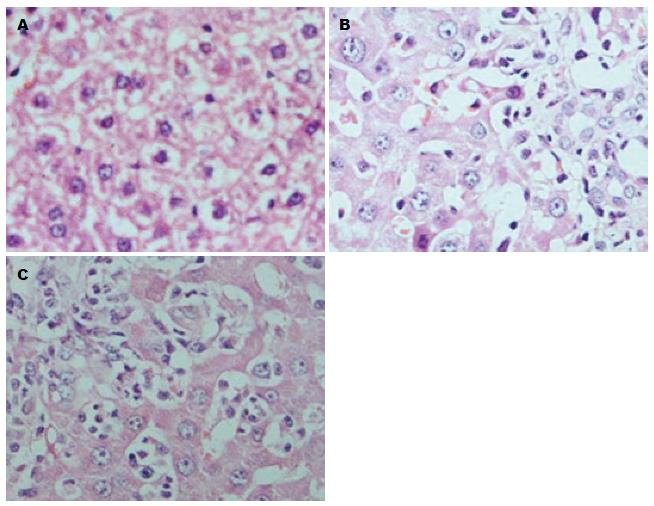
采用显微图像分析系统测量S、CLP、CLP+Dex 3组IL-6蛋白的A值. 与S组(15.28±2.87)相比, CLP组IL-6蛋白的表达明显升高(65.87±13.28), 差异具有统计学意义(P<0.01); 而CLP+Dex组(48.24±8.76)与CLP组(65.87±13.28)相比, IL-6蛋白表达呈现减低的变化趋势, 差异具有统计学意义(P<0.05). HE染色的结果也具有与此相同的趋势(图1).
CLP组与S组相比, SD大鼠肝细胞肿胀、水样变性, 少数出现点状坏死, 肝组织出现炎性细胞浸润明显增多. 而CLP+Dex组与CLP组相比, 肝组织炎性细胞浸润明显减轻(图2).
脓毒血症是机体被致病菌感染, 释放毒素进入血液, 从而激活内皮细胞等机体防御系统, 释放大量细胞因子、血小板活化因子、内皮素、花生四烯酸代谢产物、补体成分等进入全身血液循环, 引发的全身炎症反应综合征, 在神经调节的参与下, 引起多系统器官功能衰竭(multiple system organ failure, MSOF)[7]. 在Iskander等[8]的研究中, 肝功能衰竭在MSOF的顺序中排列第2位, 并且能诱发其他器官序列性的发生功能衰竭. 在本实验中, CLP组中肝功能损害的特异性指标AST、ALT较S组明显升高(168.32 U/L±38.36 U/L vs 42.16 U/L±16.52 U/L; 143.65 U/L±38.45 U/L vs 38.64 U/L±24.26 U/L), 差异有统计学意义, 也表明脓毒血症时肝脏功能出现明显损害. 非特异性炎症指标PCT、CRP已广泛用于判断感染状态, 研究结果显示PCT、CRP与脓毒血症的致死率密切相关, 并且可能与这些指标在病情进展中浓度的增幅关系密切, 目前PCT、CRP被认为是评估感染程度的重要检测指标[9,10]. 在本实验中也显示在CLP组中PCT、CRP的含量比较S组明显升高(2.68 μg/L±1.26 μg/L vs 0.28 μg/L±0.14 μg/L; 81.46 mg/L±26.58 mg/L vs 18.62 mg/L±6.28 mg/L), 差异有统计学意义.
IL-6是由212个氨基酸组成的多功能糖蛋白, 机体受炎症刺激后可诱导炎症反应, 导致免疫功能失调. IL-6蛋白在急性感染期水平显著升高, 感染控制后下降, 恢复期降至正常水平. 脓毒血症的病理过程中IL-6蛋白可促进脓毒症的进展[11]. 在本实验中, CLP组中IL-6蛋白在肝脏中的表达比S组明显增强, 也和既往报道相类似[12].
右美托咪啶是一种新型的高选择性α2肾上腺素能受体激动剂, 可以产生剂量依赖性的镇静、镇痛和抗焦虑作用. 除此以外, 右美托咪啶的其他临床作用也广受关注[13]. 近年来Sezer等[14]发现右美托咪啶对肝组织有保护作用, 实验表明在脓毒血症小鼠中使用右美托咪啶可减轻肝窦淤血及肝门炎症, 其原因可能是右美托咪啶可减少脓毒症时单核细胞和巨噬细胞产生的细胞因子, 从而减轻肝脏的损伤[15]. 本实验也发现经右美托咪啶预处理的脓毒血症SD大鼠中IL-6蛋白的表达下调, 显微观察肝组织中炎性细胞浸润明显减轻.
总之, 右美托咪啶预处理的脓毒血症SD大鼠可以通过下调IL-6蛋白的表达, 发挥抗炎作用而对脓毒血症所致急性肝脏损伤起到保护作用, 但其保护机制尚局限在动物实验阶段, 而且预处理往往不可预知, 因此, 研究脓毒血症后处理措施及有效应用于临床的治疗将是我们医务工作者努力的方向.
脓毒血症是可引起多器官功能障碍综合征(multiple organ dysfunction syndrome, MODS), 是临床中面临的难题, 脓毒症所致肝脏损伤的影响机制目前还未明确. 右美托咪啶是一种新型的高选择性α2肾上腺素能受体激动剂, 具有对重要脏器如心脏、大脑、肾脏及肺等的保护作用. 本课题通过右美托咪定预处理对脓毒症所致SD大鼠肝脏损伤的影响, 探讨其通过降低白介素-6(interleukin-6, IL-6)蛋白的水平而对肝脏产生保护的潜在机制.
王蒙, 副教授, 中国人民解放军第二军医大学附属东方肝胆外科医院肝外综合治疗一科
脓毒血症能引起MODS, 肝功能衰竭在MODS的顺序中排列第2位, 并且能诱发其他器官序列性的发生功能衰竭, 近来对脓毒血症导致MODS机制的研究有重要的临床意义.
Iskander等的研究表明肝功能衰竭在MODS的顺序中排列第2位, 并且能诱发其他器官序列性的发生功能衰竭. Pereira等研究表明IL-6蛋白可促进脓毒症的进展. Sezer等发现右美托咪啶对肝组织有保护作用.
右美托咪啶预处理的脓毒血症SD大鼠可以通过下调IL-6蛋白的表达, 发挥抗炎作用而对脓毒血症所致急性肝脏损伤起到保护作用.
明确脓毒血症对肝脏甚至多系统器官的损伤机制能为临床中的治疗提供新的途径.
脓毒血症: 指病原微生物入侵机体后所致的全身性炎症反应综合征.
本文科研性强, 有继续延伸的可能.
编辑: 韦元涛 电编:闫晋利
| 1. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [PubMed] [DOI] |
| 2. | Menda F, Köner O, Sayin M, Türe H, Imer P, Aykaç B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth. 2010;13:16-21. [PubMed] [DOI] |
| 3. | Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54:710-716. [PubMed] [DOI] |
| 4. | Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-74. [PubMed] [DOI] |
| 5. | Yang CH, Tsai PS, Wang TY, Huang CJ. Dexmedetomidine-ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation. 2009;80:1204-1210. [PubMed] [DOI] |
| 6. | Medina E. Murine model of polymicrobial septic peritonitis using cecal ligation and puncture (CLP). Methods Mol Biol. 2010;602:411-415. [PubMed] [DOI] |
| 8. | Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247-1288. [PubMed] [DOI] |
| 9. | Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 2011;26:54-64. [PubMed] [DOI] |
| 10. | Karlsson S, Heikkinen M, Pettilä V, Alila S, Väisänen S, Pulkki K, Kolho E, Ruokonen E. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care. 2010;14:R205. [PubMed] [DOI] |
| 11. | Pereira LH, Machado JR, Olegário JG, Rocha LP, Silva MV, Guimarães CS, Reis MA, Castellano LR, Ramalho FS, Corrêa RR. Interleukin-6 and C-reactive protein are overexpressed in the liver of perinatal deaths diagnosed with fetal inflammatory response syndrome. Dis Markers. 2014;2014:252780. [PubMed] [DOI] |
| 12. | Kocabaş E, Sarikçioğlu A, Aksaray N, Seydaoğlu G, Seyhun Y, Yaman A. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49:7-20. [PubMed] |
| 14. | Sezer A, Memiş D, Usta U, Süt N. The effect of dexmedetomidine on liver histopathology in a rat sepsis model: an experimental pilot study. Ulus Travma Acil Cerrahi Derg. 2010;16:108-112. [PubMed] |
| 15. | Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3-6. [PubMed] [DOI] |