修回日期: 2014-06-02
接受日期: 2014-06-06
在线出版日期: 2014-08-18
目的: 观察干扰胰岛素样生长因子-Ⅰ型受体(insulin-like growth factor-Ⅰ receptor, IGF-ⅠR)表达对肝癌(PLC/PRF/5及Bel-7404)细胞增殖、周期、凋亡的影响及联合抗癌、靶向药物抑制细胞增殖的协同作用.
方法: 设计与合成多条针对IGF-ⅠR序列的shRNA, 插入pGPU6/GFP/Neo载体, 构建、转染肝癌细胞株、筛选高效质粒, 观察沉默IGF-ⅠR表达对肝癌细胞增殖的抑制作用与机制.
结果: 4对构建IGF-ⅠR-shRNA经筛选以shRNA4干扰效果最佳且具特异性; 以shRNA4转染效率PLC/PRF/5细胞为71%和Bel-7404细胞为90%; 在mRNA水平上抑制率前者为59.6%±2.8%, 后者为54.9%±2.6%; 蛋白水平上IGF-ⅠR表达均同步减少; 转染72 h后, PLC/PRF/5细胞增殖抑制率为63.87%±3.90%(t = 19.244, P<0.001)及Bel-7404细胞为61.47%±1.70%(t = 5.493, P<0.005), 均呈时间依赖性, 且增殖周期发生G1期阻滞, 细胞周期蛋白(CyclinD1)表达受抑, 细胞凋亡增加; shRNA4增加肝癌细胞对靶向药物索拉非尼及化疗药物奥沙利铂的敏感性.
结论: 下调IGF-ⅠR基因转录可抑制肝癌细胞增殖、诱导凋亡, 改善肝癌细胞对靶向药物及化疗药物敏感性, 提示IGF-ⅠR有望成为肝癌基因治疗的有效靶点.
核心提示: 构建含有干扰作用表达载体, 以RNAi技术干扰胰岛素样生长因子-Ⅰ型受体(insulin-like growth factor-Ⅰ receptor, IGF-ⅠR), 经动物移植瘤模型研究, 探讨IGF-ⅠR干扰对肝细胞恶性转化的调控作用, 在转录水平上直接调控肝癌转移关键基因的表达, 可达到基因治疗和药物增敏效果.
引文著录: 严晓娣, 时运, 钱琦, 李景源, 陈心, 董志珍, 姚登福. shRNA干预IGF-ⅠR活化对肝癌细胞增殖的抑制作用. 世界华人消化杂志 2014; 22(23): 3396-3402
Revised: June 2, 2014
Accepted: June 6, 2014
Published online: August 18, 2014
AIM: To investigate the effect of short hairpin RNA (shRNA)-mediated silencing of insulin-like growth factor-Ⅰ receptor (IGF-ⅠR) gene transcription on cell proliferation, cell cycle progression, apoptosis and sensitivity to targeted therapy and chemotherapy in hepatocellular carcinoma (HCC) cell lines PLC/PRF/5 and Bel-7404.
METHODS: Pairs of IGF-ⅠR shRNAs were designed and synthesized based on the IGF-ⅠR sequence, and inserted into the pGPU6/GFP/Neo vector to screen the most effective one. IGF-ⅠR expression was then down-regulated with the shRNA to observe its inhibitory effect on hepatoma cell proliferation.
RESULTS: After screening, the IGF-ⅠR-shRNA4 was found to be the most efficient one for interfering IGF-ⅠR gene transcription among the 4 pairs of successfully constructed plasmids, with a transfection efficiency of 71% in PLC/PRF/5 cells and 90% in Bel-7404 cells. The expression of IGF-ⅠR mRNA was down-regulated by 59.6% ± 2.8% in PLC/PRF/5 cells and 54.9% ± 2.6% in Bel-7404 cells. After the cells was transfected with shRNA4 for 72 h, the reduced rate of cell proliferation was 61.47% ± 1.70% in Bel-7404 cells (t = 5.493, P < 0.005) and 63.87 ± 3.90% (t = 19.244, P < 0.001) in PLC/PRF/5 cells. Meanwhile, the cell cycle was arrested in the G1 phase, and the expression of Cyclin D1 was significantly down-regulated with increasing cell apoptosis. Besides, the combination of shRNA4 with sorafenib or oxaliplatin showed higher inhibitory effects on cell survival than shRNA4 alone.
CONCLUSION: Silencing IGF-ⅠR gene transcription can inhibit hepatoma cell proliferation, induce apoptosis and enhance the sensitivity to targeted therapy and chemotherapy. IGF-ⅠR may be a potential target gene for HCC gene therapy.
- Citation: Yan XD, Shi Y, Qian Q, Li JY, Chen X, Dong ZZ, Yao DF. Short hairpin RNA-mediated silencing of insulin-like growth factor-Ⅰ receptor inhibits proliferation of hepatoma cells. Shijie Huaren Xiaohua Zazhi 2014; 22(23): 3396-3402
- URL: https://www.wjgnet.com/1009-3079/full/v22/i23/3396.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i23.3396
肝细胞肝癌(hepatocellular carcinoma, HCC)是世界上最常见的恶性肿瘤之一[1,2], 他的发生发展是复杂的过程, 常涉及到癌基因激活, 抑癌基因失活和/或胚胎期某些癌基因重新复活等多信号通路和基因调控[3,4]. 胰岛素样生长因子-Ⅰ型受体(insulin-like growth factor-Ⅰ receptor, IGF-ⅠR)是细胞表面酪氨酸蛋白激酶受体[5,6], 所介导的信号转导通路在HCC的发生发展中起着重要的作用[7-9], 在胎肝和新生儿肝中大量表达, 成人阶段维持低水平[10]. 前期工作中首次以动物的肝癌模型研究证实IGF-ⅠR过表达参与肝细胞癌变, 是肝细胞恶性转化的早期标志, 干预其活化将有望成为HCC治疗的新靶点[11-13]. 本文通过构建靶向IGF-ⅠR短发夹型RNA(short hairpin RNA, shRNA)真核表达质粒, 筛选高效质粒, 转染人肝癌(Bel-7404及PLC/PRF/5)细胞株, 探讨干预IGF-ⅠR基因转录对肝癌细胞增殖的影响与机制.
PLC/PRF/5细胞株(中科院上海细胞所); Bel-7404细胞株、Annexin V-PE/7-AAD试剂盒、二甲基亚砜(dimethyl sulfoxide, DMSO)(凯基, 南京); pGPU6/GFP/Neo-shRNA、DH5α(吉玛制药, 上海); DMEM培养基、胎牛血清(Gibco, 美国); GenJet™、PolyJet™体外转录试剂(SignaGen, 美国); 细胞总蛋白抽提试剂盒、β-actin抗体、辣根过氧化物酶标记兔抗鼠IgG抗体、BCA蛋白测定试剂盒(碧云天, 上海); 鼠抗人IGF-ⅠR抗体(R&D, 美国); 鼠抗人细胞周期蛋白(CyclinD1)抗体(CST, 美国); TRIzol RNA抽提试剂、DEPC水(Invitrogen, 美国); Immobilon ECL发光液(Millipore, 美国); E.Z.N.AR Endo-free质粒纯化(OMEGA, 美国); cDNA合成(Fermentas, 美国); CCK-8试剂盒(同仁, 日本); SYBR Premix Taq(TaKaRa, 日本).
1.2.1 靶向IGF-ⅠR-shRNA质粒的构建: 按照Genbank中人IGF-ⅠR Gene ID(3480), 用Ambion公司shRNA设计系统软件设计4条shRNA. 将DNA oligo分别溶解于浓度为100 μmol/L的TE (pH 8.0), 取10×shDNA退火溶液5 μL, 正链寡核苷酸(100 nmol/L)5 μL, 反链寡核苷酸(100 nmol/L)5 μL, 双蒸水定容至50 μL退火反应体系, 95 ℃ 5 min, 产物为10 μmol/L的shRNA模板, 4 ℃保存. 另将所得模板稀释500倍用于连接反应, 终浓度为20 nmol/L. 序列如表1.
| 名称 | 正、反义链(5'→3') |
| IGF-ⅠR-shRNA1 | 5'-CACCGCTGATGTGTACGTTCCTGATTTCAAGAGAATCAGGAACGTACACATCAGCTTTTTTG-3' |
| 5'-GATCCAAAAAAGCTGATGTGTACGTTCCTGATTCTCTTGAAATCAGGAACGTACACATCAGC-3' | |
| IGF-ⅠR-shRNA2 | 5'-CACCGCGTGAGAGGATTGAGTTTCTTTCAAGAGAAGAAACTCAATCCTCTCACGCTTTTTTG-3' |
| 5'-GATCCAAAAAAGCGTGAGAGGATTGAGTTTCTTCTCTTGAAAGAAACTCAATCCTCTCACGC-3' | |
| IGF-ⅠR-shRNA3 | 5'-CACCGCAAAGTCTTTGAGAATTTCCTTCAAGAGAGGAAATTCTCAAAGACTTTGCTTTTTTG-3' |
| 5'-GATCCAAAAAAGCAAAGTCTTTGAGAATTTCCTCTCTTGAAGGAAATTCTCAAAGACTTTGC-3' | |
| IGF-ⅠR-shRNA4 | 5'-CACCGGACAGATCCTGTGTTCTTCTTTCAAGAGAAGAAGAACACAGGATCTGTCCTTTTTTG-3' |
| 5'-GATCCAAAAAAGGACAGATCCTGTGTTCTTCTTCTCTTGAAAGAAGAACACAGGATCTGTCC-3' | |
| Negative-shRNA | 5'-CACCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG-3' |
| 5'-GATCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAC-3' |
1.2.2 细胞培养和转染: 细胞株以含10%FBS的DMEM完全培养液于37 ℃含CO2培养箱培养. 细胞接种后, 融合度达95%时, 将shRNA分为干扰组、阴性对照和未处理对照组, 分别以GenJet™或PolyJet™转染试剂转染至肝癌细胞.
1.2.3 Western blot: 将Bel-7404及PLC/PRF/5细胞接种培养48 h后, 消化、收集1×106个细胞加细胞裂解液和PMSF裂解细胞, 离心, 上清液以BCA法测蛋白浓度, 每泳道加50 μg蛋白以10%SDS聚丙烯酰胺凝胶电泳, 转膜, 封闭, 鼠抗人IGF-ⅠR、CyclinD1抗体, β-actin抗体, 辣根过氧化物酶标记的兔抗鼠IgG二抗孵育, ECL显色并拍照.
1.2.4 IGF-ⅠR mRNA分析: 两种肝癌细胞株分别设转染组4组, 阴性对照组及空白对照各1组(n = 3). 转染48 h后, 以TRIzol试剂提取细胞总RNA. 分光光度计检测总RNA吸光度值及A260/280. 按试剂说明合成第一链cDNA, 根据SYBR Premix Ex Taq™Ⅱ试剂盒的说明将cDNA产物进行适当稀释后进行荧光定量PCR分析. 引物由TaKaRa公司设计合成, F: 5'-GAGGTGGCTCGGGAGAAG AT-3', R: 5'-TTCACCACACCCTTGGCAAC-3'; 内参基因GAPDH引物由逆转录试剂盒提供, F: 5'-CAAGGTCATCCATGACAACTTTG-3', R: 5'-GTCCACCACCCTGTTGCTGTAG-3'. 步骤如下: 95 ℃ 30 s; 94 ℃ 5 s, 60 ℃ 45 s, 40个循环; 4 ℃保存. 以Ct-比较法对IGF-ⅠR基因相对定量: Ratio = 2-∆∆Ct(∆Ct = CtIGF-ⅠR-CtGAPDH), ∆∆Ct = ∆Ct样本-∆Ct空白), 相对表达量 = 2-∆∆Ct.
1.2.5 CCK-8法检测细胞增殖、药物敏感性及双染检测细胞凋亡: 转染48 h后, 96孔板中加入100 μL的细胞悬液(已转染或药物处理), 分别设置24、48及72 h, 每组设立3个复孔, 每组含调零、空白对照、阴性对照及有效干扰孔, 37 ℃培养箱孵育适当时间, 弃培养液, 加入新鲜完全培养液, 并向每孔加入10 μL CCK-8溶液, 继续培养4 h, 于酶标仪450 nm处测吸光度值. 转染后培养72 h, 消化、离心, 收集细胞, 加入500 μL结合缓冲液悬浮细胞, 再加入1 μL Annexin V-PE混匀, 室温避光反应15 min; 最后加入5 μL 7-ADD, 轻轻混匀, 常温避光反应15 min, 流式细胞仪检测分析结果.
1.2.6 细胞周期分析: Bel-7404及PLC/PRF/5细胞株分别转染48 h后, 换新鲜完全培养液, 培养24 h弃完全培养液, PBS洗涤2次; 胰酶消化, 收集细胞于离心管中, 预冷PBS洗2次, 弃PBS; 加入1 mL预冷700 mL/L乙醇, 轻轻吹匀细胞, 4 ℃固定24 h; 1000 g×5 min离心弃上清, PBS洗涤1次; 加入0.5 mL碘化丙啶染色, 37 ℃避光静置30 min, 流式细胞仪488 nm处检测红色荧光, 分析结果.
统计学处理 结果以mean±SD表示, 统计数据用Graphpad Prism5.0和SPSS18.0软件进行绘图和统计分析, 多个样本均数间的比较先行方差齐性检验, 再行方差分析, 两样本均数的比较采用t检验, 样本率之间的比较采用Fisher确切概率法, 相关性用等级相关分析. P<0.05为差异有统计学意义.
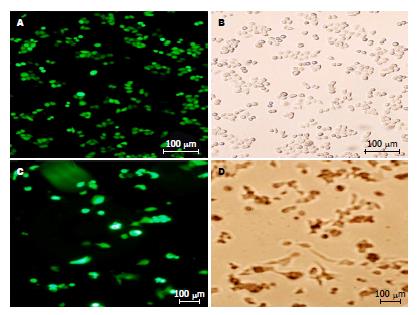
由pGPU6/GFP/Neo标记的4条IGF-ⅠR-shRNA重组干扰质粒成功转染至人肝癌Bel-7404和PLC/PRF/5细胞株, 在荧光倒置显微镜下观察结果如图1. 图中带荧光的细胞即为转染成功的肝癌细胞, 随机选取上中下左右5个细胞视野的细胞计算转染效率(Bel-7404细胞达90%, PLC/PRF/5细胞达71%).
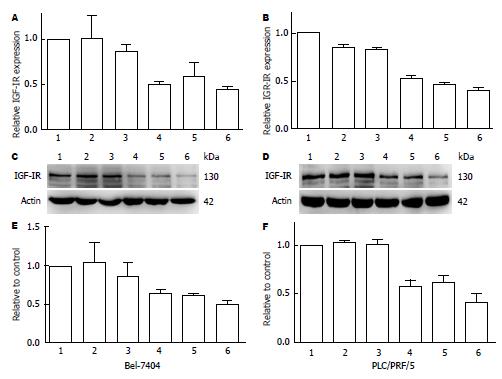
FQ-RT-PCR检测4条shRNA转染48 h对Bel-7404及PLC/PRF/5细胞中IGF-ⅠR mRNA表达抑制, 其中以IGF-ⅠR-shRNA4效果最佳, 抑制率分别为54.9%±2.6%和59.6%±2.8%(n = 3)(图2A, B); Western blot显示干扰质粒能抑制两种人肝癌细胞中的IGF-ⅠR蛋白表达(图2C, D), 将其表达进行半定量分析, 证实以IGF-ⅠR-shRNA4效果最佳(抑制率为49.4%±8.4%和58.3%±9.4%, n = 3)(图2E, F). 因此, IGF-ⅠR- shRNA4被选为后续研究.
shRNA4转染Bel-7404及PLC/PRF/5细胞后, CCK-8法分析细胞增殖的变化. 24 h和48 h干扰组细胞较阴性对照组均明显抑制(P<0.001), 72 h时抑制率最高, 分别为61.47%±1.70%(t = 5.493, P<0.005), 63.87%±3.90%(t = 19.244, P<0.001). 流式细胞仪分析shRNA4干扰Bel-7404及PLC/PRF/5细胞IGF-ⅠR表达对细胞凋亡的影响, 干扰组细胞凋亡率(分别为35.96%、44.84%)显著高于空白(12.16%、6.62%)及阴性对照组(9.43%、4.02%, P<0.001).
流式细胞仪分析可见两种细胞中干扰组G0/G1期细胞百分率分别为59.02%±1.30%、65.39%±0.52%, 明显高于对照组48.38%±0.81%(t = 12.032, P<0.001)、53.49%±0.74%(t = 22.789, P<0.001); Western blot显示CyclinD1表达, 转染组分别为59.61%±4.69%和39.91%±0.47%, 明显低于阴性对照组分别为90%±3.44%(t = 7.389, P<0.05)、90.17%±14.57%(t = 4.876, P<0.05).
shRNA4转染Bel-7404及PLC/PRF/5细胞48 h后, 分别加入不同浓度的索拉非尼(0.0、2.5、5.0、10.0、20.0和40.0 nmol/L)处理24 h, shRNA4与索拉非尼联合应用对细胞增殖的抑制作用通过CCK-8检测, 索拉非尼对肝癌细胞存活和生长的抑制作用在一定范围内呈浓度依赖性方式, 浓度在20 nmol/L时显著抑制细胞增殖(表2). 索拉非尼与shRNA4联合对细胞存活和生长抑制更明显(n = 3, P<0.05).
| 分组(nmol/L) | 对照组 | Neg-shRNA | shRNA4 plus Sorafenib | t值 | P值 | |||||
| Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | |
| 0 | 0.297±0.06 | 0.373±0.02 | 0.310±0.07 | 0.336±0.06 | 0.199±0.07 | 0.199±0.07 | 2.377 | -2.39 | 0.045 | -0.044 |
| 2.5 | 0.326±0.10 | 0.360±0.08 | 0.335±0.11 | 0.325±0.10 | 0.188±0.00 | 0.158±0.02 | 3.086 | -2.450 | 0.002 | -0.040 |
| 5 | 0.337±0.11 | 0.351±0.05 | 0.318±0.05 | 0.324±0.11 | 0.191±0.06 | 0.178±0.05 | 2.606 | -2.447 | 0.031 | -0.040 |
| 10 | 0.331±0.01 | 0.331±0.10 | 0.284±0.17 | 0.284±0.07 | 0.152±0.09 | 0.152±0.09 | 4.42 | -1.977 | 0.002 | -0.048 |
| 20 | 0.093±0.03 | 0.093±0.03 | 0.054±0.02 | 0.054±0.02 | 0.033±0.03 | 0.033±0.03 | 4.45 | -3.162 | 0.002 | -0.013 |
| 40 | 0.000±0.00 | 0.003±0.00 | 0.000±0.00 | 0.002±0.00 | 0.003±0.00 | 0.003±0.00 | - | - | - | - |
降低IGF-ⅠR的表达可增加Bel-7404及PLC/PRF/5对化疗药物奥沙利铂的敏感性. 奥沙利铂(0、5、10、20和40 μmol/L)处理肝癌细胞(表3), 随浓度增加, 对肝癌细胞增殖抑制作用明显; 相同浓度的奥沙利铂加入空白, 阴性对照及shRNA转染组, 发现与空白及阴性对照组相比, 转染组细胞增殖的抑制作用更加明显(n = 3, P<0.05).
| 分组(nmol/L) | 对照组 | Neg-shRNA | shRNA4 plus Sorafenib | t值 | P值 | |||||
| PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | PLC/PRF/5 | Bel-7404 | |
| 0.0 | 2.391±0.30 | 2.929±0.08 | 2.351±0.17 | 2.662±0.29 | 1.429±0.07b | 2.291±0.11 | 6.983 | 10.489 | <0.001 | <0.001 |
| 5.0 | 1.064±0.21 | 2.006±0.29 | 1.014±0.05 | 1.879±0.11 | 0.677±0.08b | 1.636±0.30 | 3.851 | 1.983 | 0.005 | 0.083 |
| 10.0 | 0.659±0.16 | 1.670±0.25 | 0.575±0.03 | 1.431±0.12 | 0.417±0.13a | 1.034± 0.18b | 2.625 | 4.617 | 0.030 | 0.002 |
| 20.0 | 0.288±0.12 | 1.178±0.13 | 0.280±0.05 | 1.179±0.15 | 0.168±0.02 | 0.655±0.09b | 2.206 | 7.396 | 0.059 | <0.001 |
| 40.0 | 0.164±0.04 | 0.247±0.03 | 0.162±0.01 | 0.319±0.06 | 0.099±0.04a | 0.145±0.03b | 2.569 | 5.376 | 0.033 | 0.001 |
IGF-ⅠR与HCC的发生发展及转移密切相关[14,15], IGF-ⅠR与相关配体结合后, 启动信号通路[16], 将细胞生长增殖信号持续传至细胞核[17], 使细胞周期调节失控, 加速细胞从G1期进入S期, 促进有丝分裂[18]、细胞表型恶性转化、抗凋亡和诱导血管内皮生长因子(vascular endothelial growth factor, VEGF)表达等, 促进肝癌的发生发展[19-21]. IGF-ⅠR作为肝癌靶向治疗的新靶点, 具有应用前景[22,23]. RNA干扰技术能特异性下调相关基因的表达, 达到有效地基因沉默, 被广泛应用于肿瘤的治疗中[24-27]. 本文构建靶向IGF-ⅠR的真核表达载体质粒, 转染至IGF-ⅠR表达较高的HBV阳性PLC/PRF/5及HBV阴性Bel-7404肝癌细胞株. 经qRT-PCR及Western blot证实IGF-ⅠR表达明显受到抑制, 选择最有效的shRNA4用于后续研究.
shRNA4分别转染Bel-7404及PLC/PRF/5细胞, 分析空白、阴性对照和干扰组细胞增殖的变化, 干扰组细胞较阴性对照组均明显抑制, 72 h时效果最明显, 呈时间依赖性, 抑制率分别为61.5%、63.9%; 观察转染后对细胞凋亡影响, 证实干扰组细胞凋亡率显著高于空白及阴性对照组; 流式细胞仪分析两种细胞中干扰组G0/G1期细胞百分率分别为59.0%、65.49%明显高于阴性对照组的48.4%、53.5%, 提示shRNA能使肝癌细胞滞于有丝分裂前期; CyclinD1是细胞周期中G1-S转化的关键调节者, 具有严格的周期顺序性, Western blot分析显示shRNA转染组CyclinD1表达明显低于对照组, shRNA干预影响细胞周期.
HCC的治疗方法比较局限, 对放疗及化疗不敏感, 尤其是晚期患者. 目前, 索拉非尼是唯一能够有效提高患者生存率的药物[28], 但对于索拉非尼无效, 确诊时已经错过手术最佳时期者, 现在还没有标准的治疗方法. 本研究采用多靶向治疗评估对HCC细胞的影响, 显示索拉非尼、奥沙利铂分别联合基因干预IGF-ⅠR的表达后对HCC细胞增殖抑制率明显高于单独作用, 提示抑制IGF-ⅠR的表达, 能够增加肝癌细胞对靶向药物及化疗药物的敏感性. 总之, 干预IGF-ⅠR活化转录, 将有助于肝癌诊断、发病机制探讨及在肝癌分子靶向治疗方面具有开发前景.
胰岛素样生长因子-Ⅰ型受体(insu-lin-like growth factor-Ⅰ receptor, IGF-ⅠR)与相关配体结合后, 启动信号通路, 将细胞生长增殖信号持续传至细胞核, 使细胞周期调节失控, 促进有丝分裂、恶性转化、抗凋亡和诱导血管内皮生长因子表达等, 促进肝癌的发生发展.
范学工, 教授, 中南大学湘雅医院感染病科.
IGF-ⅠR胎肝和新生儿肝中过表达, 为细胞表面酪氨酸蛋白激酶受体, 介导信号转导通路在肝细胞性肝癌(hepatocellular carcinoma, HCC)的发生发展中起着重要的作用, 该受体在正常肝组织中未见明显表达, 但是否可成为HCC治疗的新靶点值得研究.
利用肝细胞恶性转化的动物模型观察, 在肝细胞出现颗粒样变性、不典型增生到癌变形成过程中, 显示IGF-ⅠR表达呈进行性梯度增加, 肝细胞恶性转化组明显高于对照组、变性组和癌前组, 肝、血中两者呈显著正相关.
分子靶向干预是以肝癌细胞过表达的某些标志性分子为靶点, 选择针对性的阻断剂, 有效地干预该信号通路, 达到抑制肝细胞恶性转化效果. 肝癌组织IGF-ⅠR过表达, 其活化抑制有望成为HCC基因治疗的新方法. 国内、外尚未见诸于文献.
采用多靶向治疗评估对HCC细胞的影响, 显示索拉非尼、奥沙利铂分别联合基因干预IGF-ⅠR的表达后对HCC细胞增殖抑制率明显高于单独作用, 抑制IGF-ⅠR表达增加肝癌细胞对靶向药物及化疗药物敏感性. 干预IGF-ⅠR活化转录有助于肝癌诊断、发病机制探讨及在分子靶向方面具有开发前景.
本文设计合理, 具有较好应用价值.
编辑 田滢 电编 都珍珍
| 1. | Aino H, Sumie S, Niizeki T, Kuromatsu R, Tajiri N, Nakano M, Satani M, Yamada S, Okamura S, Shimose S. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol Clin Oncol. 2014;2:393-398. [PubMed] |
| 2. | Liu Y, Wang X, Li S, Hu H, Zhang D, Hu P, Yang Y, Ren H. The role of von Willebrand factor as a biomarker of tumor development in hepatitis B virus-associated human hepatocellular carcinoma: A quantitative proteomic based study. J Proteomics. 2014;106:99-112. [PubMed] [DOI] |
| 3. | Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799-806. [PubMed] [DOI] |
| 4. | Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, Ahamed K, Bonora M, Hoyeau N, Fléjou JF. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One. 2012;7:e48071. [PubMed] [DOI] |
| 5. | Xue M, Cao X, Zhong Y, Kuang D, Liu X, Zhao Z, Li H. Insulin-like growth factor-1 receptor (IGF-1R) kinase inhibitors in cancer therapy: advances and perspectives. Curr Pharm Des. 2012;18:2901-2913. [PubMed] [DOI] |
| 6. | Escobar S, Fuentes EN, Poblete E, Valdés JA, Safian D, Reyes AE, Alvarez M, Molina A. Molecular cloning of IGF-1 and IGF-1 receptor and their expression pattern in the Chilean flounder (Paralichthys adspersus). Comp Biochem Physiol B Biochem Mol Biol. 2011;159:140-147. [PubMed] [DOI] |
| 7. | Ubagai T, Kikuchi T, Fukusato T, Ono Y. Aflatoxin B1 modulates the insulin-like growth factor-2 dependent signaling axis. Toxicol In Vitro. 2010;24:783-789. [PubMed] [DOI] |
| 8. | Weng CJ, Hsieh YH, Tsai CM, Chu YH, Ueng KC, Liu YF, Yeh YH, Su SC, Chen YC, Chen MK. Relationship of insulin-like growth factors system gene polymorphisms with the susceptibility and pathological development of hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1808-1815. [PubMed] [DOI] |
| 9. | Abou-Alfa GK, Capanu M, O'Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319-324. [PubMed] [DOI] |
| 10. | Dong Z, Yao M, Wang L, Yan X, Gu X, Shi Y, Yao N, Qiu L, Wu W, Yao D. Abnormal expression of insulin-like growth factor-I receptor in hepatoma tissue and its inhibition to promote apoptosis of tumor cells. Tumour Biol. 2013;34:3397-3405. [PubMed] [DOI] |
| 12. | Yan XD, Yao M, Wang L, Zhang HJ, Yan MJ, Gu X, Shi Y, Chen J, Dong ZZ, Yao DF. Overexpression of insulin-like growth factor-I receptor as a pertinent biomarker for hepatocytes malignant transformation. World J Gastroenterol. 2013;19:6084-6092. [PubMed] [DOI] |
| 14. | Yao NH, Yao DF, Dong ZZ, Yan XD, Chen J, Yao M, Wang L, Yan MJ. [Effects of inhibited IGF-IR expression on proliferation and apoptosis of human hepatocellular carcinoma cell lines]. Zhonghua Ganzangbing Zazhi. 2013;21:376-380. [PubMed] |
| 15. | Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res. 2012;72:3-12. [PubMed] [DOI] |
| 16. | Chen C, Zhang Y, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel bispecific antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor. Mol Cancer Ther. 2014;13:90-100. [PubMed] [DOI] |
| 17. | Gómez-Hernández A, Escribano Ó, Perdomo L, Otero YF, García-Gómez G, Fernández S, Beneit N, Benito M. Implication of insulin receptor A isoform and IRA/IGF-IR hybrid receptors in the aortic vascular smooth muscle cell proliferation: role of TNF-α and IGF-II. Endocrinology. 2013;154:2352-2364. [PubMed] [DOI] |
| 18. | Svendsen AM, Winge SB, Zimmermann M, Lindvig AB, Warzecha CB, Sajid W, Horne MC, De Meyts P. Down-regulation of cyclin G2 by insulin, IGF-I (insulin-like growth factor 1) and X10 (AspB10 insulin): role in mitogenesis. Biochem J. 2014;457:69-77. [PubMed] [DOI] |
| 19. | Yao WF, Liu JW, Sheng GL, Huang DS. Blockade of IGF-IR exerts anticancer effects in hepatocellular carcinoma. Mol Med Rep. 2011;4:719-722. [PubMed] [DOI] |
| 20. | Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011;39:524-543. [PubMed] |
| 21. | Kang GH, Lee BS, Lee ES, Kim SH, Lee HY, Kang DY. Prognostic significance of p53, mTOR, c-Met, IGF-1R, and HSP70 overexpression after the resection of hepatocellular carcinoma. Gut Liver. 2014;8:79-87. [PubMed] [DOI] |
| 22. | Yue L, Wang Y, Wang H, Gao H, Liang J, Sui A, Xiang J, Zhou F, Xu C, Zhao W. Inhibition of hepatocellular carcinoma cell growth by an anti-insulin-like growth factor-I receptor monoclonal antibody. Oncol Rep. 2012;28:1453-1460. [PubMed] [DOI] |
| 23. | Zhang YW, Yan DL, Wang W, Zhao HW, Lu X, Wu JZ, Zhou JR. Knockdown of insulin-like growth factor I receptor inhibits the growth and enhances chemo-sensitivity of liver cancer cells. Curr Cancer Drug Targets. 2012;12:74-84. [PubMed] |
| 24. | Segrestaa JM, Petrescou L, Julien D, Bugard P. [Methodology for the study of a hypnotic by a double-blind technic]. Therapie. 1977;32:459-470. [PubMed] [DOI] |
| 25. | Tang J, Li J, Zeng G, Tang Y, Tian W, He J, York JP, Xia X. Antisense oligonucleotide suppression of human IGF-1R inhibits the growth and survival of in vitro cultured epithelial ovarian cancer cells. J Ovarian Res. 2013;6:71. [PubMed] [DOI] |
| 26. | Shtyren MIa. [Venous pathomorphology in occlusive lesions of the arteries of the lower extremities]. Arkh Patol. 1990;52:36-38. [PubMed] [DOI] |
| 27. | Corvaia N, Beck A, Caussanel V, Goetsch L. Insulin-like growth factor receptor type I as a target for cancer therapy. Front Biosci (Schol Ed). 2013;5:439-450. [PubMed] |
| 28. | Jo S, Shim HK. A patient who has survived for a long period with repeated radiotherapies for multifocal extrahepatic metastases from hepatocellular carcinoma. Radiat Oncol J. 2013;31:267-272. [PubMed] |