修回日期: 2014-04-21
接受日期: 2014-04-28
在线出版日期: 2014-07-08
目的: 研究碳水化合物反应元件结合蛋白转录因子(carbohydrate responsive element binding protein, ChREBP)及其靶基因含patatin样磷脂酶域3(patatin-like phospholipase domain containing family member A3, PNPLA3)在非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)中的作用以及NAFLD的发病机制.
方法: 将48只200 g±20 g体质量的成年♂SD大鼠, 随机分为对照组24只(普通饲料喂养)和模型组24只(高脂饲料喂养), 分别于第4周末, 第8周末和第12周末处死各组大鼠8只, 并进行血清生化的测定; 在光镜下观察肝组织病理变化; 用免疫组织化学方法检测ChREBP蛋白的表达水平; 用RT-PCR技术检测肝脏ChREBP以及PNPLA3基因的mRNA的表达.
结果: (1)成功构建NAFLD大鼠模型, 光镜下观察可见对照组大鼠肝脏组织结构正常, 各项生化指标均在正常范围, 肝组织内表达少量ChREBP蛋白; (2)模型组大鼠较肥胖, 肝脏明显增大, 肝细胞内可见脂滴沉积, 血清生化天门冬氨酸氨基转移酶(aspartate aminotransferase, AST)、丙氨酸氨基转移酶(alanine aminotransferase, ALT)、总胆固醇(total cholesterol, TC)、甘油三酯(triglycerides, TG)水平升高(P<0.05), 空腹血糖(fasting blood glucose, FBG)及空腹血胰岛素(fasting serum insulin, FINS)水平升高(P<0.05), 并随着喂养时间的增加而逐渐增加(P<0.05), 而胰岛素敏感指数(insulin sensitive index, ISI)明显下降(P<0.05); (3)模型组大鼠肝组织ChREBP蛋白和ChREBP、PNPLA3 mRNA的表达水平升高, 与对照组差异具有统计学意义(P<0.05), 且随造模时间的延长其表达量也增加(P<0.05); (4)相关分析显示, 在模型组中, ChREBP mRNA的相对表达量与PNPLA3、血清AST 、ALT水平、肝脏炎症活动度计分呈正相关(r = 0.409, P<0.05; r = 0.498, P<0.05; r = 0.677, P<0.01; r = 0.729, P<0.01; r = 0.566, P<0.01), 与ISI呈负相关(r = -0.611, P<0.01).
结论: 高脂饮食引起ChREBP的表达增强, 其可能激活了脂质合成相关基因PNPLA3的表达, 并可能通过参与胰岛素抵抗的调控促进脂肪肝的形成.
核心提示: 碳水化合物反应元件结合蛋白转录因子(carbohydrate responsive element binding protein)及其靶基因磷脂酶域3(patatin-like phospholipase domain containing family member A3)在非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)发病中起着非常重要的作用, 可以利用他们与NAFLD的关系, 阻止NAFLD发生发展的过程, 延缓疾病的进展, 以期待对NAFLD的治疗有新的方法.
引文著录: 季玲, 李昌平. ChREBP及其靶基因PNPLA3在大鼠非酒精性脂肪性肝病发病中的作用. 世界华人消化杂志 2014; 22(19): 2679-2685
Revised: April 21, 2014
Accepted: April 28, 2014
Published online: July 8, 2014
AIM: To explore the role of carbohydrate responsive element binding protein (ChREBP) and its target gene patatin-like phospholipase domain containing family member A3 (PNPLA3) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD).
METHODS: Forty-eight adult male rats were randomly and equally divided into two groups to receive a normal fed diet (control group) or a high-fat diet (NAFLD model group). At the end of the 4th, 8th and 12th wk, 8 rats in each group were killed to determine serum biochemical parameters, observe pathological changes in hepatic tissue under a light microscope, detect the expression of ChREBP protein by immunohistochemistry, and detect the expression of ChREBP and PNPLA3 mRNAs in the liver by RT-PCR.
RESULTS: NAFLD was successfully induced in rats after feeding a high-fat diet. In the control group, rat liver lobule structure was intact under a light microscope, the biochemical parameters were in the normal ranges, and expression of ChREBP protein was weak in the liver tissue. Compared with the control group, rat weight was increased, the volume of the liver was obviously increased, fat drop deposition was visible in liver cells, and the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), fasting blood glucose (FBG), and fasting serum insulin (FINS) were significantly elevated in the model group (P < 0.05 for all). With the prolongation of feeding time, blood AST, ALT, TC, TG FBG and FINS levels were gradually elevated and ISI significantly declined (P < 0.05). ChREBP protein expression and ChREBP and PNPLA3 mRNA expression in rat liver tissue were increased in the model group compared with the control group (P < 0.05). With the prolongation of feeding time, ChREBP protein expression and ChREBP and PNPLA3 mRNA expression were significantly increased (P < 0.05). ChREBP mRNA expression in the model group was positively correlated with PNPLA3 expression, serum levels of AST, ALT, and hepatic inflammatory activity score (r = 0.409, P < 0.05; r = 0.498, P < 0.05; r = 0.677, P < 0.01; r = 0.729, P < 0.01; r = 0.566, P < 0.01), but had a negative correlation with ISI (r = -0.611, P < 0.01).
CONCLUSION: High fat diet induces the expression of ChREBP, which may activate the expression of lipid synthesis related gene PNPLA3 and promote the formation of fatty liver through regulation of insulin resistance.
- Citation: Ji L, Li CP. Role of ChREBP and its target gene PNPLA3 in pathogenesis of nonalcoholic fatty liver disease in rats. Shijie Huaren Xiaohua Zazhi 2014; 22(19): 2679-2685
- URL: https://www.wjgnet.com/1009-3079/full/v22/i19/2679.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i19.2679
非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)是一种无过量饮酒史, 但肝病理学改变与酒精性肝病相似的临床病理综合征. NAFLD包括非酒精性单纯性脂肪肝(nonalcoholic simple fatty liver, NAFL)、非酒精性脂肪性肝炎(nonalcoholic steatohepatitis, NASH)、NASH相关肝纤维化和肝硬化[1]. 近年来随着人们生活水平和饮食习惯的改变, 其患病率也不断的攀升[2], 已然在我国成为第2大肝病[3]. 据统计NAFLD在我国约占15%[4], 在肥胖人群中高达75%[5]. NAFLD可通过非酒精性单纯性脂肪肝向非酒精性脂肪性肝炎甚至肝硬化发展[6-8], 所以NAFLD受到了越来越多的重视. 其具体发病机制到目前仍然尚不完全清楚, 而有关NAFLD发病机制大多数人较为认可Day等[9]提出的"二次打击"理论, 其中胰岛素抵抗更被认为中心环节. 本实验主要是通过检测碳水化合物反应元件结合蛋白转录因子(carbohydrate responsive element binding protein, ChREBP)及其靶基因PNPLA3在非酒精性脂肪性肝病模型中的表达以及与胰岛素抵抗的关系, 进一步研究NAFLD的发病机制.
实验大鼠由简阳达硕动物科技有限公司提供, 普通饲料由泸州医学院实验动物中心提供, 高脂饲料(基础饲料82.5%+猪油10%+胆酸钠0.5%+胆固醇2%+蔗糖5%)由泸州医学院实验动物中心加工. ChREBP抗体购自博奥森科技有限公司; EnVisionTM试剂盒购自DaKo生物科技有限公; DAB显色试剂盒购自上海博谷生物科技有限公司; RT-PCR试剂盒购自成都生物技术公司; DNA Marker购自天根生化科技有限公司公司; TRIzol试剂和焦碳酸乙二脂购自Invitrogen公司; RNA抽提试剂盒购自天根生化科技有限公司.
1.2.1 建立NAFLD模型: 将体质量为200 g±20 g的48只健康♂SD大鼠, 适应性喂养1 wk后随机分为正常对照组和模型组, 每组24只. 正常对照组大鼠给予普通饲料喂养, 模型组给予高脂饲料喂养, 实验中大鼠自由饮水和进食, 并观察大鼠的一般情况, 分别于第4、8和12周末随机选取各组中大鼠8只处死, 常规制备血清和肝脏组织石蜡切片, 并保留少量肝组织用于mRNA的检测.
1.2.2 血清生化指标的检测: 采用全自动生化分析仪测定丙氨酸氨基转移酶(alanine aminotransferase, ALT)、天门冬氨酸氨基转移酶(aspartate aminotransferase, AST)、甘油三酯(triglycerides, TG)、总胆固醇(total cholesterol , TC)、空腹血糖(fasting blood glucose, FBG); 放免法测定血清空腹胰岛素(fasting serum insulin, FINS)水平; 并计算胰岛素敏感指数ISI = ln[1/(FINS×FBG)].
1.2.3 肝组织病理学: 将取出的肝脏组织固定于4%的多聚甲醛中24 h后逐级的进行乙醇脱水、二甲苯透明、浸蜡, 最后石蜡包埋切片, 行常规 HE染色, 在光镜下观察肝组织病理变化. 石蜡切片行ChREBP的Envision免疫组织化学染色. 按照EnVisionTM二步法免疫组织化学试剂盒说明书进行, 一抗的浓度为1:100. 以正常的肝组织作为阳性对照, 用PBS液替代一抗作为阴性空白对照, DAB显色, 苏木素复染, 中性树胶脂封片. 采用Imagepro plus图像分析软件分析, 计算累积光密度值.
1.2.4 逆转录聚合酶连反应(RT-PCR): 采用TRIzol法提取肝脏组织的总RNA, 依照试剂盒提供的说明书进行逆转录反应. PCR引物序列: PNPLA3: 上游引物: 5'-GTGCTGGTGTCTGATTTCCG-3', 下游引物: 5'-CCCACTGTAGATGCCGTTCT-3'; ChREBP: 上游引物: 5'-AAAGGCCTCAAGTTGCTATG-3', 下游引物: 5'-AGACAACAGCCTCAGGTTTC-3'. PCR反应条件: 预变性(PNPLA3: 94 ℃ 5 min, ChREBP: 94 ℃ 5 min), 变性(PNPLA3: 94 ℃ 35 s, ChREBP: 94 ℃ 3 s), 退火(PNPLA3: 57 ℃ 40 s, ChREBP: 56 ℃ 35 s), 延伸(PNPLA3: 72 ℃ 35 s, ChREBP: 72 ℃ 30 s), 30个循环后于72 ℃延伸5 min. GADPH上游引物: 5'-GACCCCTTCATTGACCTCAAC-3', 下游引物: 5'-CGCTCCTGGAAGATGGTGAT-3', PCR反应条件: 预变性94 ℃ 5 min, 变性94 ℃ 30 s, 退火 55 ℃ 30 s, 延伸72 ℃ 30 s, 28个循环后于72 ℃延伸5 min; 产物分析: 将扩增产物加入1.5%的琼脂糖凝胶电泳, 以DNA Marker为分子量参照标准, 凝胶成像系统下观察记录结果, 并用图像分析软件对得到的条带进行灰度扫描, 目标基因mRNA的相对含量用目标基因条带光密度/GAPDH基因条带光密度.
统计学处理 采用SPSS13.0软件进行统计学分析, 计量资料用mean±SD表示, 采用t检验(independent-samples t Test)或方差齐性检验后采用单因素方差分析(one-way ANOVA), 两两比较采用LSD-t检验, 参数间进行Pearson相关分析. P<0.05为差异有统计学意义.
与正常对照组比较, 非酒精性肝病模型组血清ALT、AST、TC、TG均明显上升(P<0.05), 且随造模时间的延长, ALT、AST、TC、TG也逐渐升高(P<0.05)(表1).
| 分组 | AST(U/L) | ALT(U/L) | TC(mmol/L) | TG(mmol/L) |
| 4 wk末 | ||||
| 对照组 | 54.76±8.51 | 37.43±4.15 | 0.96±0.32 | 0.67±0.32 |
| 模型组 | 62.89±6.41a | 49.68±6.99b | 1.43±0.42a | 1.01±0.24a |
| 8 wk末 | ||||
| 对照组 | 54.74±8.01 | 39.45±7.74 | 1.25±0.33 | 0.65±0.22 |
| 模型组 | 98.56±7.83bd | 69.99±9.88bd | 2.16±0.61bc | 1.68±0.41bd |
| 12 wk末 | ||||
| 对照组 | 94.64±13.97 | 43.59±9.20 | 1.50±0.56 | 0.69±0.22 |
| 模型组 | 136.51±6.83bd | 93.17±11.83bd | 3.38±0.62bd | 2.52±0.51bd |
与正常对照组比较, 非酒精性肝病模型组血清FBG、FINS均明显上升(P<0.05), ISI均明显下降(P<0.05), 且随造模时间的延长, FBG、FINS也逐渐升高(P<0.05)(表2).
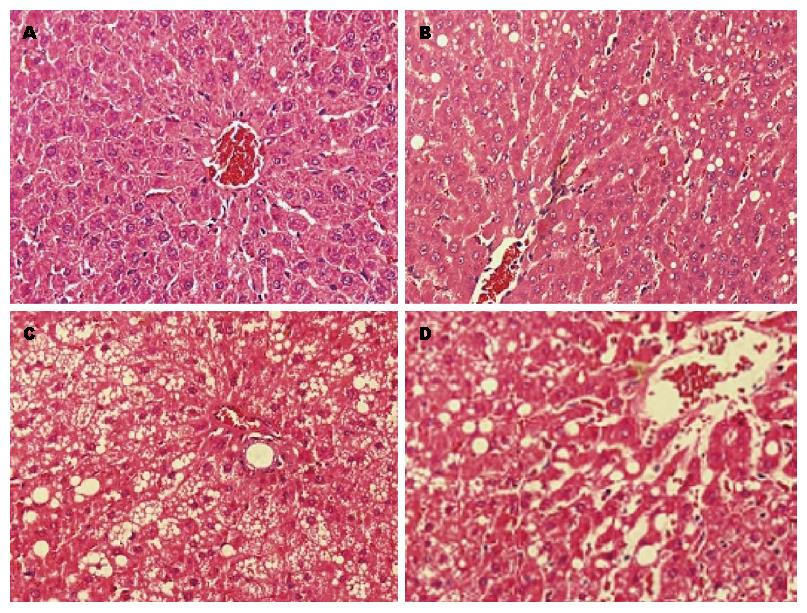
HE染色光镜下观察显示: 正常对照组大鼠肝组织的形态学表现正常, 肝小叶结构完整, 肝索排列整齐围绕中央静脉呈放射状分布. 4 wk末模型组大鼠可见肝细胞轻度散在的脂肪空泡伴轻度水肿, 肝小叶结构无明显紊乱, 无炎细胞浸润. 8 wk末模型组大鼠可见肝小叶结构紊乱, 肝细胞肿胀, 肝小叶内可见少量炎细胞浸润, 较多的肝细胞充满脂滴, 以大泡性脂肪变为主, 较大的脂滴可将细胞核挤到细胞的一侧. 12 wk末模型组大鼠可见广泛的肝细胞脂肪变性, 胞质疏松, 呈气球样改变, 小叶结构明显紊乱, 肝索排列紊乱, 小叶内和汇管区炎细胞不同程度浸润, 可见局灶性坏死(图1).
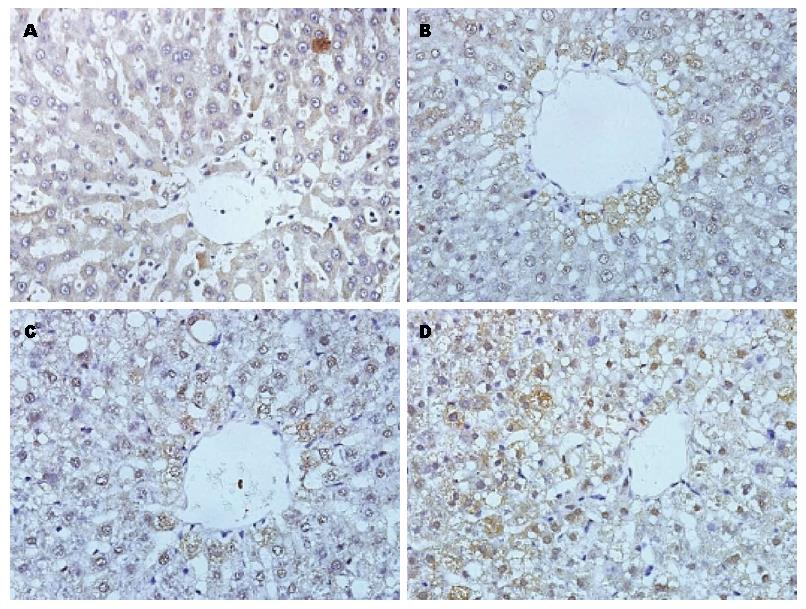
ChREBP蛋白主要在中央静脉周围及汇管区表达, 正常对照组ChREBP表达于胞质中, 为散在分布的浅棕黄色颗粒. NAFLD组4 wk时ChREBP表达与正常对照组相比表达增强. 8 wk时ChREBP表达明显增强, 细胞质中棕黄色颗粒增多, 同时少数肝细胞核出现棕黄色阳性颗粒; 随着肝细胞脂肪变程度加重, 在12 wk时ChREBP表达更强, 细胞质中可见大量棕褐色颗粒沉积, 同时大部分细胞核也呈阳性着色(图2), 所以在模型组大鼠肝细胞内, 细胞核中的ChREBP水平较正常对照组大鼠增加. 第4、8、12周大鼠与同期对照组比较, 模型组肝组织ChREBP蛋白表达显著升高(P<0.05); 且随喂养周期增长而显著升高(P<0.05)(表3).
ChREBP和PNPLA3 mRNA的表达分别以ChREBP或PNPLA3/GAPDH的基因条带光密度比值作为ChREBP和PNPLA3 mRNA表达的相对含量. NAFLD组与正常对照组相比ChREBP和PNPLA3 mRNA表达增多, 并随脂肪肝程度的加重逐渐增加, 与正常组相比有显著性差异(P<0.05)(表4, 表5).
在模型组中, ChREBP mRNA的相对表达量与PNPLA3、血清AST、ALT水平、肝脏炎症活动度计分呈正相关(r = 0.409, P<0.05; r = 0.498, P<0.05; r = 0.677, P<0.01; r = 0.729, P<0.01; r = 0.566, P<0.01), 与ISI呈负相关(r = - 0.611, P<0.01).
NAFLD是遗传、代谢等因素相关的一种疾病[10], 其发病机制非常复杂, 由多个相关病理生理因素组成[11], 而由Day等[12]提出的氧化应激、脂质过氧化损伤的"二次打击"学说是较被公认的学说, 其中胰岛素抵抗被认为在NAFLD的发病中起比较重要的作用. 许多研究证实NAFLD患者存在比较明显的IR[13-16]. 胰岛素抵抗时外周脂肪组织分解释放游离脂肪酸(free fatty acid, FFA)增多, 进入肝脏后使肝内脂肪蓄积、甘油三酯合成增多, 最后形成NAFLD[17]. 而升高的FFA又会增加胰岛素抵抗[18,19], 形成恶性循环. 所以, 胰岛素抵抗促进NAFLD的发生发展[20-24].
ChREBP属于转录因子家族[25], 是由864个氨基酸组成的碱性螺旋-环-螺旋/亮氨酸拉链(bHLH/ZIP)大分子DNA结合蛋白, 是葡萄糖过程中的关键转录因子[26], 主要与糖脂代谢和IR有关[27], 通过激活其靶基因PNPLA3引起肝脏内脂肪存积[28]. 本实验用高脂饮食成功复制了非酒精性脂肪肝模型, 结果可见大鼠肝脏形成了不同程度的肝脏脂肪变, 肥胖体型、高脂血症、高胰岛素血症以及胰岛素抵抗伴转氨酶增高. 通过与对照组进行相关指标的观测和比较, 发现正常组大鼠表达少量的ChREBP蛋白, 模型组ChREBP mRNA及蛋白表达量、PNPLA3 mRNA的表达量增加, 之间呈相同变化趋势, 并与血清AST、ALT水平呈正相关, 与ISI呈负相关. 与相关研究一致, 正常情况下大鼠肝脏表达很少量的ChREBP, 当喂养高脂饮食时, 其表达就会明显增高, 即经过长期高脂饮食的喂养可引起大鼠肝脏ChREBP mRNA的表达升高, 并可能通过上调PNPLA3等成脂基因的表达[29-32], 参与触发胰岛素抵抗的调控损伤脂肪细胞对胰岛素的反应性, 使外周大量脂肪动员, 血中不断增多的游离脂肪酸运往肝脏, 破坏了肝脏内脂肪的动态平衡, 并刺激微粒体脂质过氧化使肝脏发生氧化应激, 而导致糖脂代谢失衡, 甘油三酯合成增多, 使肝脏内脂肪积聚, 引起NAFLD.
ChREBP在肝脏的糖脂代谢中起着非常重要的作用, 相关研究表明敲除ChREBP基因的小鼠体质量明显下降, 代谢综合症状和胰岛素抵抗也明显改善, 所以ChREBP可能成为治疗非酒精性脂肪性肝病等代谢综合征的新靶点. 虽然关于非酒精性脂肪性肝病的发病的研究一直在进行, 但是其发病机制至今仍未完全清楚, 非酒精性脂肪性肝病的早期预防和治疗又非常重要, 所以需要进一步了解其发病机制, 改善脂肪肝并延缓疾病的进展. 对ChREBP基因及其靶基因PNPLA3在非酒精性脂肪性肝病发病中的作用的深入研究, 有望为非酒精性脂肪性肝病的临床预防和治疗提供新的理论依据.
非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)现已成为常见肝病之一, 现今越来越多的人患有NAFLD, 严重危害着人们的生命健康. 因此阐明NAFLD的发病机制, 对NAFLD的预防和诊治具有重要的理论价值和临床价值.
孙学英, 教授, 哈尔滨医科大学第一附属医院
近年较多研究发现碳水化合物反应元件结合蛋白转录因子(carbohydrate responsive element binding protein, ChREBP)及其靶基因含patatin样磷脂酶域3(patatin-like phospholipase domain containing family member A3, PNPLA3)可以增加NAFLD的发病易感性, 与胰岛素抵抗、肝炎的发生发展密切联系, 参与NAFLD的发病机制, 但其具体发病机制至今仍不完全清楚.
Dentin等研究敲除ChREBP基因的小鼠发现, 基因敲除后小鼠的胰岛素敏感性增加, 表明ChREBP与胰岛素抵抗(insulin resistance)有关, 而抑制肝脏ChREBP可以纠正小鼠脂肪肝和葡萄糖耐受情况, 成为解决肥胖、脂肪肝等一系列健康问题的又一途径.
本文探讨ChREBP及其靶基因PNPLA3在NAFLD中的作用, 大鼠肝脏ChREBP mRNA的表达升高, 可能通过上调其靶基因PNPLA3等成脂基因的表达, 通过触发胰岛素抵抗的调控参与高脂饮食大鼠非酒精性脂肪性肝病的形成.
对ChREBP基因及其靶基因PNPLA3在非酒精性脂肪性肝病发病中的作用的深入研究, 进一步了解其发病机制, 改善脂肪肝并延缓疾病的进展, 有望为非酒精性脂肪性肝病的临床预防和治疗提供新的理论依据.
本文有一定科学意义, 具有一定指导意义.
编辑: 田滢 电编:鲁亚静
| 2. | Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol. 2002;33:1098-1104. [PubMed] |
| 3. | Manco M, Bottazzo G, DeVito R, Marcellini M, Mingrone G, Nobili V. Nonalcoholic fatty liver disease in children. J Am Coll Nutr. 2008;27:667-676. [PubMed] |
| 4. | Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, Dai F, Li F, Chen SY. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825-1832. [PubMed] |
| 5. | Kirovski G, Schacherer D, Wobser H, Huber H, Niessen C, Beer C, Schölmerich J, Hellerbrand C. Prevalence of ultrasound-diagnosed non-alcoholic fatty liver disease in a hospital cohort and its association with anthropometric, biochemical and sonographic characteristics. Int J Clin Exp Med. 2010;3:202-210. [PubMed] |
| 6. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [PubMed] |
| 7. | Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97-113. [PubMed] [DOI] |
| 8. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [PubMed] [DOI] |
| 9. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [PubMed] |
| 10. | Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682-689. [PubMed] |
| 11. | Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009;25:230-237. [PubMed] |
| 12. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [PubMed] |
| 16. | Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295-300. [PubMed] [DOI] |
| 17. | Duez H, Lamarche B, Valéro R, Pavlic M, Proctor S, Xiao C, Szeto L, Patterson BW, Lewis GF. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation. 2008;117:2369-2376. [PubMed] [DOI] |
| 20. | Zhou WD, Yang YL, Che ZH. [Non-alcoholic fatty liver, high sensitivity C reactive protein and insulin resistance]. Zhongnan Daxue Xuebao Yixueban. 2008;33:565-570. [PubMed] |
| 21. | Sakurai M, Takamura T, Ota T, Ando H, Akahori H, Kaji K, Sasaki M, Nakanuma Y, Miura K, Kaneko S. Liver steatosis, but not fibrosis, is associated with insulin resistance in nonalcoholic fatty liver disease. J Gastroenterol. 2007;42:312-317. [PubMed] |
| 22. | Park SH, Kim BI, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Kim H, Keum DK. Body fat distribution and insulin resistance: beyond obesity in nonalcoholic fatty liver disease among overweight men. J Am Coll Nutr. 2007;26:321-326. [PubMed] |
| 23. | Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125-1149, ix. [PubMed] |
| 24. | Petrides AS, Schulze-Berge D, Vogt C, Matthews DE, Strohmeyer G. Glucose resistance contributes to diabetes mellitus in cirrhosis. Hepatology. 1993;18:284-291. [PubMed] |
| 25. | Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006;55:1179-1189. [PubMed] |
| 28. | Perttilä J, Huaman-Samanez C, Caron S, Tanhuanpää K, Staels B, Yki-Järvinen H, Olkkonen VM. PNPLA3 is regulated by glucose in human hepatocytes, and its I148M mutant slows down triglyceride hydrolysis. Am J Physiol Endocrinol Metab. 2012;302:E1063-E1069. [PubMed] [DOI] |
| 29. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [PubMed] [DOI] |
| 30. | Johansson LE, Hoffstedt J, Parikh H, Carlsson E, Wabitsch M, Bondeson AG, Hedenbro J, Tornqvist H, Groop L, Ridderstråle M. Variation in the adiponutrin gene influences its expression and associates with obesity. Diabetes. 2006;55:826-833. [PubMed] |
| 31. | Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107-110. [PubMed] |