修回日期: 2014-04-04
接受日期: 2014-04-20
在线出版日期: 2014-06-18
目的: 探讨糖基化终末产物(advanced glycation end products, AGEs)对糖尿病(diabetes mellitus, DM)大鼠结肠动力及肌球蛋白轻链激酶(myosin light chain kinase, MLCK)表达的影响.
方法: SD大鼠48只, 随机均分为4组, 即正常对照组、正常+AGEs抑制剂氨基胍(aminoguanidine, AG)干预组、DM组、DM+AG干预组. 单次腹腔注射链脲佐菌素(streptozotocin, STZ)(60 mg/kg)构建DM大鼠模型, 造模成功后, AG干预按1 g/L饮用水给药, 持续至实验结束. ELISA、Western blot检测大鼠血清及结肠肌层AGEs水平, 玻璃珠排出法检测结肠转运功能, MPA分析系统检测结肠环肌肌条收缩力, Western blot检测结肠肌层肌球蛋白轻链激酶(myosin light chain kinase, MLCK)的表达. 各组间比较采用单因素方差分析和成组t检验.
结果: 与正常对照组相比, DM大鼠结肠肌层AGEs表达增多, 血清AGEs水平升高(3.00 μg/mL±0.34 μg/mL vs 4.31 μg/mL±0.73 μg/mL, P<0.01), 结肠转运时间延长(209.50 min±48.88 min vs 297.00 min±35.71 min, P<0.01), 环肌肌条收缩力减弱(2.17 g±0.57 g vs 0.80 g±0.34 g, P<0.01), 结肠肌层MLCK蛋白表达减少(1.46±0.11 vs 1.01±0.08, P<0.01). AG干预不影响大鼠血糖水平, 但与DM组相比, DM+AG组大鼠结肠AGEs表达减少, 血清AGEs水平降低(4.31 μg/mL±0.73 μg/mL vs 3.35 μg/mL±0.58 μg/mL, P<0.05), 结肠转运时间缩短(297.00 min±35.71 min vs 212.13 min±42.95 min, P<0.01)、肌条收缩力增强(0.80 g±0.34 g vs 1.49 g±0.44 g, P<0.01), 结肠肌层MLCK蛋白表达升高(1.01±0.08 vs 1.40±0.09, P<0.01).
结论: AGEs可能通过减少结肠肌层MLCK蛋白表达参与DM大鼠结肠动力障碍的发生.
核心提示: 本研究提示糖尿病(diabetes mellitus, DM)大鼠血清及结肠肌层糖基化终末产物(advanced glycation end products)的含量均上升, 他可能是通过减少DM大鼠结肠肌层肌球蛋白轻链激酶(myosin light chain kinase)表达参与其结肠动力障碍的发生.
引文著录: 王庆娥, 王云, 朱滢, 孙晓萌, 林琳. 糖基化终末产物影响结肠平滑肌肌球蛋白轻链激酶表达在糖尿病大鼠结肠动力障碍中的作用. 世界华人消化杂志 2014; 22(17): 2427-2433
Revised: April 4, 2014
Accepted: April 20, 2014
Published online: June 18, 2014
AIM: To investigate whether advanced glycation end products (AGEs) affect colonic motility and the expression of myosin light chain kinase in diabetic rats.
METHODS: Forty-eight SD rats were evenly and randomly divided into four groups: normal controls, normal rats treated with aminoguanidine (AG, an inhibitor of AGEs), diabetic rats, and diabetic rats treated with AG. Diabetes mellitus (DM) was induced by a single intraperitoneal injection of streptozotocin (STZ) (60 mg/kg). AG was given in drinking water at a dose of 1 g/L after DM was induced. ELISA and Western blot were used to measure levels of AGEs in serum and the colonic muscle layer, respectively. Colon transit time was measured by glass bead expulsion test, and the contractility of circular smooth muscle strips was tested using the MPA analysis system. The protein expression level of myosin light chain kinase (MLCK) was determined by Western blot.
RESULTS: Compared with the normal control group, the expression levels of AGEs in the colonic muscle layer and serum (3.00 μg/mL ± 0.34 μg/mL vs 4.31 μg/mL ± 0.73 μg/mL, P < 0.01) were increased, colon transit time was delayed (209.50 min ± 48.88 min vs 297.00 min ± 35.71 min, P < 0.01), the contractility of circular smooth muscle strips was weakened (2.17 g ± 0.57 g vs 0.80 g ± 0.34 g, P < 0.01), and the expression of MLCK in the colonic muscle layer was lower (1.46 ± 0.11 vs 1.01 ± 0.08, P < 0.01) in the diabetic group. There was no significant change in blood glucose levels in rats treated with AG. Compared with the diabetic group, DM rats treated with AG showed decreased levels of AGEs in the colon muscle layer and serum (4.31 μg/mL ± 0.73 μg/mL vs 3.35 μg/mL ± 0.58 μg/mL, P < 0.05), shortened colon transit time (297.00 min ± 35.71 min vs 212.13 min ± 42.95 min, P < 0.01), enhanced contractility of circular smooth muscle strips (0.80 g ± 0.34 g vs 1.49 g ± 0.44 g, P < 0.01), and higher expression of MLCK in the colonic muscle layer (1.01 ± 0.08 vs 1.40 ± 0.09, P < 0.01).
CONCLUSION: AGEs might be involved in the pathogenesis of colonic motility dysfunction in diabetic rats via decreasing the expression of MLCK.
- Citation: Wang QE, Wang Y, Zhu Y, Sun XM, Lin L. Effect of advanced glycation end products on myosin light chain kinase expression in smooth muscle cells and colonic motility dysfunction in diabetic rats. Shijie Huaren Xiaohua Zazhi 2014; 22(17): 2427-2433
- URL: https://www.wjgnet.com/1009-3079/full/v22/i17/2427.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i17.2427
结肠动力障碍是糖尿病(diabetic metabolism, DM)患者常见的消化系并发症, 发病率约45%, 便秘和腹泻为常见症状[1,2]. 发病机制尚不明确. 研究认为高水平氧化应激、胃肠神经病变、Cajal间质细胞丢失、平滑肌病变等与之相关[3-5]. 糖基化终末产物(advanced glycation end products, AGEs)是由蛋白质、脂质或核酸等物质通过非酶糖基化反应, 自发与葡萄糖或其他还原单糖反应生成的稳定共价化合物[6], Nε-羧甲基赖氨酸(Nε-carboxymethyllysine, CML)是AGEs重要组成成分, 可用于反映体内AGEs的含量[6,7]. 氨基胍(aminoguanidine, AG)是AGEs抑制剂, 用于DM慢性并发症的许多研究中[8-10]. 本文探讨AGEs是否影响DM大鼠结肠动力, 为DM胃肠动力障碍的研究提供依据.
健康8周龄♂SD大鼠(北京维通利华实验动物技术有限公司)48只, 饲养于南京医科大学动物中心(清洁级), 所有动物实验均遵循南京医科大学动物伦理条例. 乙酰胆碱(Sigma-Aldrich, 美国), 链脲佐菌素(Sigma-Aldrich, 美国), 氨基胍(Sigma-Aldrich, 美国), CML ELISA试剂盒(CircuLex公司, 日本), 肌球蛋白轻链激酶(myosin light chain kinase, MLCK)抗体(Abcam, 美国), RIPA裂解液(碧云天生物技术研究所, 中国上海), MPA分析系统(上海奥尔科特生物技术有限公司, 可用于记录分析胃电图、肠电图、胃肠张力、活动节律等), 血糖仪(Roche, 瑞士), 余试剂均为市售分析纯.
1.2.1 建立DM大鼠模型及AG给药干预: 48只SD大鼠随机均分为4组, 分别为正常对照组、正常+AGEs抑制剂氨基胍(aminoguanidine, AG)干预组、DM组、DM+AG干预组. 所有大鼠禁食12 h后, DM组给予单次腹腔注射链脲佐菌素(streptozotocin, STZ)60 mg/kg造模, 对照组则给予等体积柠檬酸溶剂腹腔注射. 3 d后尾静脉取血, 罗氏血糖仪检测血糖, 血糖≥16.7 mmol/L且持续1 wk, 认为DM模型建立成功. 造模成功后, 正常+AG组及DM+AG组开始给予AG(1 g/L饮用水)干预, 正常对照组及DM组则给予正常饮用水.
1.2.2 ELISA检测大鼠血清中CML含量: 各组大鼠于第16周时眼眶取血, 离心取血清, 按照CML ELISA试剂盒说明书, 将1:4稀释的待检样品加入已包被的反应孔中, 每个样本做双复孔, 室温震荡孵育1 h; 洗涤4次, 加入二抗, 室温震荡孵育1 h; 洗涤4次, 加入底物反应液, 避光、室温震荡孵育20 min; 加入反应中止液, 将板置入酶标仪内, 在450 nm波长下读板.
1.2.3 玻璃珠排出法检测结肠转运功能[11,12]: 各组大鼠饲养至第15周进行, 实验前禁食12 h, 乙醚吸入短暂麻醉后, 用8号导尿管将直径5 mm的玻璃珠自肛门推入3 cm深处, 缓慢退出导尿管, 使玻璃珠滞留于结肠内. 置入玻璃珠的大鼠单独饲养于一个笼子, 进食及饮水如常, 记录各实验大鼠排出玻璃珠所需的时间, 即为结肠转运时间. 饲养过程中DM组死亡4只大鼠, 因此其余各组均随机选取8只大鼠进行实验.
1.2.4 MPA分析系统检测肌条收缩功能: 各组大鼠饲养至第16周时, 断颈处死, 取出距肛门5 cm处结肠, 剪取约2 cm长结肠组织, 放入用95%O2、5%CO2饱和过的Kreb's液中, 沿肠管横轴剪取约2 mm×8 mm的肌条, 即为环肌肌条, 丝线结扎肌条两端, 一端固定于组织固定器, 另一端与传感器力臂相连, 记录肌肉收缩张力变化. 待记录线稳定后, 予不同浓度的乙酰胆碱(acetylcholine, Ach)促发肌条收缩, 观察肌条收缩张力变化情况.
1.2.5 Western blot检测结肠肌层CML及MLCK表达: 结肠组织均于第16周时取自各组大鼠, 玻片刮去黏膜层及浆膜, 加入液氮研钵中磨成粉状, RIPA裂解液提取肌层组织蛋白, BCA法测定蛋白浓度, 十二烷基硫酸钠(SDS)-聚丙烯酰胺凝胶恒流30 mA电泳, 恒压80 V转膜, 5%脱脂奶粉室温封闭1.5 h, 加入稀释的CML一抗、MLCK一抗或GAPDH一抗. 4 ℃过夜, TBST洗膜3次, 加入二抗, 37 ℃温育2 h, 再用TBST洗膜3次, 加显色液, 曝光显影, 以GAPDH为内参, 凝胶成像分析系统处理数据.
统计学处理 应用SPSS17.0统计软件进行分析, 所有数据以mean±SD表示, 各组间比较采用单因素方差分析和成组t检验. P<0.05为差异有统计学意义.
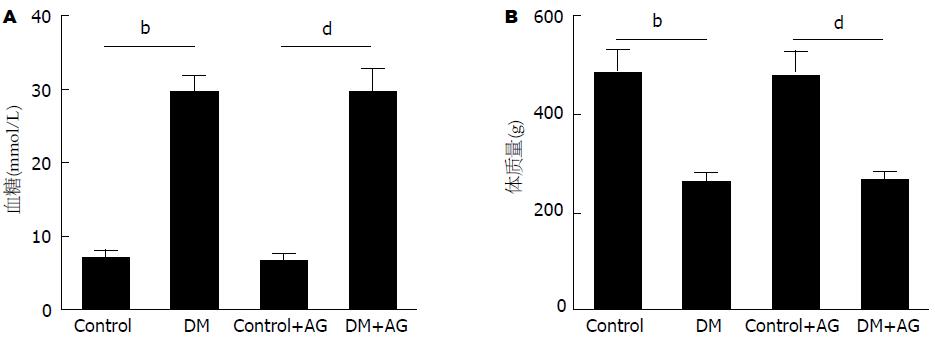
在第0(造模前)、1、4、8、16周时分别检测大鼠血糖、体质量, 结果显示, 与正常对照组相比, DM大鼠血糖显著升高、体质量逐渐下降, 持续至第16周(6.83 mmol/L±0.87 mmol/L vs 29.35 mmol/L±1.97 mmol/L; 484.20 g±43.63 g vs 261.30 g±18.26 g; 均P<0.01). 给予AG干预后, DM大鼠仍保持较高血糖水平, 亦持续至第16周(6.67 mmol/L±0.63 mmol/L vs 29.52 mmol/L±2.87 mmol/L, P<0.01), 且体质量并未上升(476.00 g±48.40 g vs 265.10 g±14.91 g, P<0.01). 此结果说明, DM大鼠模型构建成功, AG干预不影响大鼠血糖及体质量(图1).
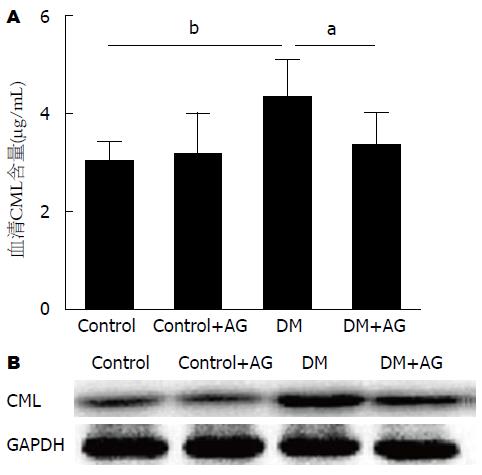
检测结果为, DM大鼠血清CML水平显著高于正常对照组(3.00 μg/mL±0.34 μg/mL vs 4.31 μg/mL±0.73 μg/mL, P<0.01). AG干预后, DM大鼠血清中CML水平降低(4.31 μg/mL±0.73 μg/mL vs 3.35 μg/mL±0.58 μg/mL, P<0.05)(图2A). Western blot检测各组大鼠结肠肌层CML的表达, 其结果与血清CML水平一致(图2B). 所以, AG干预可有效抑制DM大鼠血清及结肠组织中AGEs的合成.
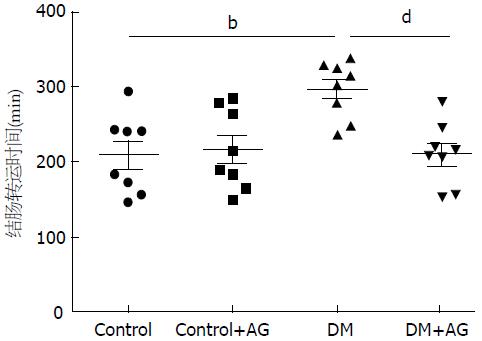
与正常对照组相比, DM大鼠结肠转运时间明显延长(209.50 min±48.88 min vs 297.00 min±35.71 min, P<0.01). 而DM+AG组结肠转运时间短于DM组(212.13 min±42.95 min vs 297.00 min±35.71 min, P<0.01)(图3).
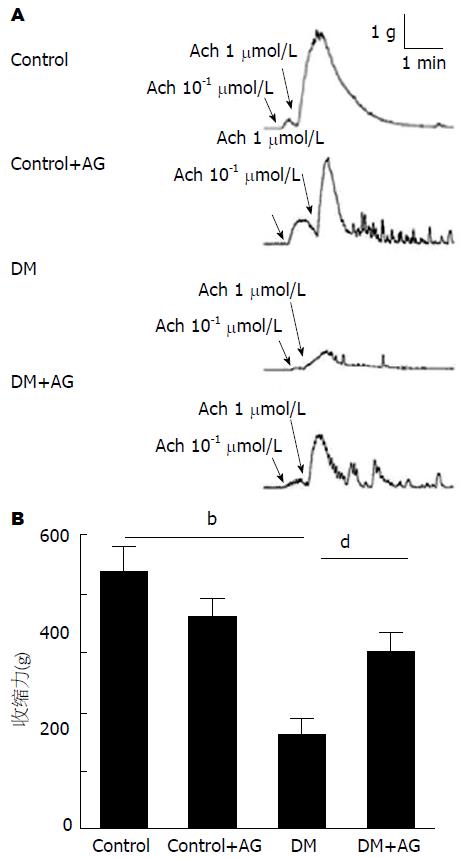
在10-1 μmol/L及1 μmol/L浓度Ach的作用下, DM大鼠肌条均表现为收缩能力减弱, AG干预后其收缩能力有所提高(图4A). 在1 μmol/L Ach作用时, DM大鼠肌条的最大收缩张力较对照组明显降低(2.17 g±0.57 g vs 0.80 g±0.34 g, P<0.01). AG干预的DM大鼠肌条收缩能力较DM大鼠增强(0.80 g±0.34 g vs 1.49 g±0.44 g, P<0.01)(图4B).
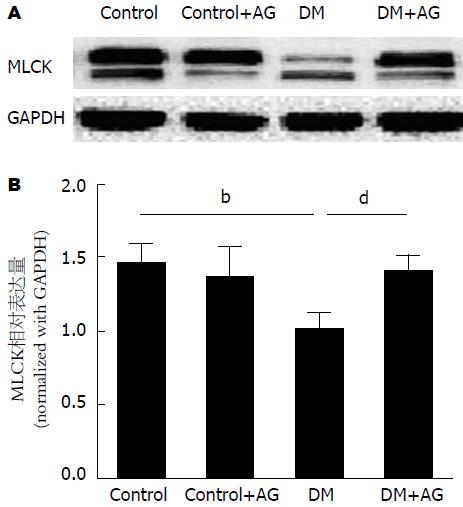
与对照组相比, DM大鼠结肠肌层MLCK表达下降, AG干预后其表达升高(1.46±0.11、1.01±0.08 vs 1.40±0.09, 均P<0.01)(图5).
AGEs是葡萄糖与蛋白质、脂质、核酸等物质经非酶糖基化反应形成的一类复合物, 其生成过程复杂, 主要包括3个步骤: 首先, 葡萄糖与上述物质的氨基酸残基结合, 形成Schiff碱基, 若此反应过程中葡萄糖浓度下降, 则该反应可逆; 然后, Schiff碱基发生化学结构重组, 形成Amadori产物; 第3阶段即Amadori产物持续堆积、经过一系列化学结构重组并与蛋白质交联, 最终形成稳定的AGEs, 该过程一般需数周. CML则是AGEs的主要成分, 可反映AGEs含量, 其分子量约10-12 kDa[13,14]. AG通过清除上述反应过程中生成的羰基产物, 抑制AGEs合成[15]. 本研究中DM大鼠高血糖持续16 wk, 足以形成不可逆的AGEs. 研究结果也显示, DM大鼠血清、结肠肌层AGEs水平均显著高于对照组, 但给予AG干预后, AGEs水平明显降低, 提示本实验所采用的AG剂量可有效抑制AGEs的形成.
AGEs在组织和器官中的蓄积参与DM慢性并发症的发生, 包括视网膜病变、肾脏病变、动脉粥样硬化等[16-18]. 但其在DM胃肠病变中的作用仅有部分报道. DM过程中, AGEs可引起小肠刷状缘理化特征改变[19], 抑制肠神经元一氧化氮合酶表达[20,21]. 亦有研究指出, AGEs或其受体存在于DM大鼠(Wistar大鼠) 空肠、回肠及结肠[22]. 但AGEs对DM结肠动力的影响尚未见报道. 本研究发现, DM大鼠结肠转运时间较正常组明显延长, 结肠环肌肌条收缩力降低, 提示DM大鼠存在结肠动力障碍. 这些现象伴随着血清、结肠肌层组织中AGEs水平的升高, 且给予AGEs抑制剂AG后, 上述病变均得到有效改善. 提示AGEs可能参与DM结肠动力障碍的发生.
本研究还显示, DM大鼠结肠肌层MLCK表达下降. MLCK是平滑肌收缩过程中的重要信号分子[23]. 平滑肌细胞受到相应刺激(如细胞因子、神经递质、胃肠激素等)后, 引起胞内钙离子浓度升高, 钙离子和钙调蛋白结合, 此复合物结合并活化MLCK, 继而磷酸化肌球蛋白轻链(myosin light chain, MLC), 促进肌球蛋白与肌动蛋白结合, 产生横桥周期, 引起平滑肌收缩[24]. 所以, MLCK表达下降可降低MLC磷酸化水平, 使平滑肌收缩功能受损, 最终引起胃肠动力障碍. Hu等[25]亦指出, DM大鼠幽门、回肠MLCK表达下降参与DM胃肠动力障碍的发生. 本研究对DM大鼠给予AG干预后, 其结肠动力有所恢复, MLCK表达亦升高. 因此推测, AGEs可能下调MLCK表达而影响DM结肠动力.
总之, AGEs参与DM大鼠结肠动力障碍的发生, MLCK表达下降是可能的机制之一. 研究报道, AGEs可通过受体依赖型和非受体依赖型两条通路参与DM合并症[26,27], 前者通过与细胞外特异性受体结合激活胞内信号通路而改变细胞功能, 后者通过非酶糖化反应、形成蛋白质交叉连接改变细胞外基质的结构, 促进合并症的发生[28,29]. 本研究未对SD大鼠结肠是否表达AGEs受体进行探讨, 现有研究结果亦不能说明AGEs通过何种途径影响DM大鼠的结肠动力. 因此, AGEs发挥作用的具体机制如何、AGEs对影响平滑肌收缩的其他因素有何作用, 仍待研究.
糖尿病(diabetes mellitus, DM)胃肠动力障碍主要表现为胃肠排空延迟, 胃肠自主神经病变、Cajal间质细胞丢失、平滑肌病变等与之相关. 平滑肌细胞保持正常收缩功能是胃肠运动的基础, 肌球蛋白轻链激酶(myosin light chain kinase, MLCK)与平滑肌收缩密切相关, 其表达减少是本病的病理基础之一.
宁钧宇, 副研究员, 北京市疾病预防控制中心卫生毒理所
糖基化终末产物(advanced glycation end products, AGEs)在DM患者血清及多种组织中含量均显著升高, AGEs在DM肾病、DM血管病变中的研究颇多. 但其在DM结肠平滑肌病变中的作用鲜有报道.
Pengmin Chen等学者报道AGEs或其受体存在于DM大鼠空肠、回肠及结肠. Wenchao等研究发现, DM大鼠幽门、回肠肌球蛋白轻链激酶(myosin light chain kinase, MLCK)表达下降参与DM胃肠动力障碍的发生.
本文首次报道了糖基化终末产物(advanced glycation end products, AGEs)影响DM大鼠结肠平滑肌myosin light chain kinase, MLCK表达在DM结肠动力障碍中的作用, 为DM胃肠动力障碍的研究提供依据.
本研究显示, AGEs可能通过减少结肠肌层MLCK表达参与DM大鼠结肠动力障碍的发生, 为DM胃肠动力障碍发病机制的研究提供新思路.
本文采用动物模型研究AGEs在DM结肠动力障碍中的作用及可能机制, 创新性较好. 具有一定的理论及应用价值.
编辑: 田滢 电编:鲁亚静
| 1. | Forrest A, Parsons M. The enhanced spontaneous activity of the diabetic colon is not the consequence of impaired inhibitory control mechanisms. Auton Autacoid Pharmacol. 2003;23:149-158. [PubMed] [DOI] |
| 2. | Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71-76. [PubMed] |
| 3. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-138, e26. [PubMed] [DOI] |
| 4. | Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666-670. [PubMed] [DOI] |
| 5. | Forrest A, Huizinga JD, Wang XY, Liu LW, Parsons M. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315-G326. [PubMed] [DOI] |
| 6. | Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129-146. [PubMed] |
| 7. | Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, Laakso M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007;50:1409-1417. [PubMed] |
| 8. | Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31-40. [PubMed] [DOI] |
| 9. | Hughes JM, Kuiper EJ, Klaassen I, Canning P, Stitt AW, Van Bezu J, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia. 2007;50:1089-1098. [PubMed] |
| 10. | Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes. 2007;56:1825-1833. [PubMed] [DOI] |
| 11. | Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34-G40. [PubMed] |
| 12. | Poole DP, Pelayo JC, Cattaruzza F, Kuo YM, Gai G, Chiu JV, Bron R, Furness JB, Grady EF, Bunnett NW. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141:565-575, 575.e1-e4. [PubMed] [DOI] |
| 13. | Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247-1265. [PubMed] [DOI] |
| 14. | Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011;7:526-539. [PubMed] [DOI] |
| 15. | Nagai R, Shirakawa J, Ohno R, Moroishi N, Nagai M. Inhibition of AGEs formation by natural products. Amino Acids. 2014;46:261-266. [PubMed] [DOI] |
| 16. | Sugiyama T, Okuno T, Fukuhara M, Oku H, Ikeda T, Obayashi H, Ohta M, Fukui M, Hasegawa G, Nakamura N. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85:406-412. [PubMed] [DOI] |
| 17. | Fukami K, Yamagishi S, Ueda S, Okuda S. Role of AGEs in diabetic nephropathy. Curr Pharm Des. 2008;14:946-952. [PubMed] [DOI] |
| 18. | Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20:177-183. [PubMed] [DOI] |
| 19. | Bhor VM, Sivakami S. Regional variations in intestinal brush border membrane fluidity and function during diabetes and the role of oxidative stress and non-enzymatic glycation. Mol Cell Biochem. 2003;252:125-132. [PubMed] |
| 20. | Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392-400. [PubMed] [DOI] |
| 21. | Jeyabal PV, Kumar R, Gangula PR, Micci MA, Pasricha PJ. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil. 2008;20:253-261. [PubMed] |
| 22. | Chen P, Zhao J, Gregersen H. Up-regulated expression of advanced glycation end-products and their receptor in the small intestine and colon of diabetic rats. Dig Dis Sci. 2012;57:48-57. [PubMed] [DOI] |
| 23. | Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20 Suppl 1:39-53. [PubMed] |
| 24. | Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633-645. [PubMed] [DOI] |
| 25. | Hu W, Feng P. Myosin light chain kinase is involved in the mechanism of gastrointestinal dysfunction in diabetic rats. Dig Dis Sci. 2012;57:1197-1202. [PubMed] [DOI] |
| 26. | Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521-1528. [PubMed] [DOI] |
| 27. | Vlassara H. Receptor-mediated interactions of advanced glycosylation end products with cellular components within diabetic tissues. Diabetes. 1992;41 Suppl 2:52-56. [PubMed] [DOI] |
| 28. | Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl). 2005;83:876-886. [PubMed] [DOI] |
| 29. | Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1-15. [PubMed] |