修回日期: 2014-04-13
接受日期: 2014-04-17
在线出版日期: 2014-06-08
目的: 观察以短发夹RNA(short hairpin RNA, shRNA)下调磷脂酰肌醇蛋白多糖-3(glypican-3, GPC-3)基因转录, 对抑制肝癌MHCC-97H细胞侵袭和血管新生的作用与机制.
方法: 将对GPC-3特异性shRNA转染肝癌MHCC-97H细胞, 以荧光定量PCR和Western blot分析GPC-3 mRNA及蛋白表达; 以5-ethynyl-2'-deoxyuridine(EdU)和Transwell法等评估细胞增殖、迁移和侵袭能力.
结果: shRNA1转染MHCC-97H细胞, GPC-3在mRNA水平下降75.6%(t = 15.473, P<0.001), 蛋白表达同步下调; 干预GPC-3基因转录, 可抑制肝癌细胞增殖, 细胞迁移与侵袭能力减低, 与抗癌药物具协同作用; Wnt/β-catenin中β-catenin表达下调67.7%; Hedgehogs通路中脑胶质瘤相关癌基因1(glioma-associated oncogene 1, Gli1)表达上调53.3%; shRNA1转染72 h细胞血管内皮生长因子(vascular endothelial growth factor, VEGF)表达下调54.2%(t = 46.746, P<0.001).
结论: shRNA下调GPC-3转录, 经Wnt/β-catenin和Hh信号通路抑制癌细胞迁移、侵袭和血管新生, 为肝癌基因治疗潜在的分子靶目标.
核心提示: 以特异性短发夹RNA(short hairpin RNA, shRNA)沉默磷脂酰肌醇蛋白多糖-3(glypican-3, GPC-3)基因转录, 分析Wnt/β-catenin通路β-catenin和Hedgehogs通路中脑胶质瘤相关癌基因1(glioma-associated oncogene 1, Gli1)表达的作用, 干预GPC-3转录抑制肝癌细胞侵袭和血管新生.
引文著录: 邰伯军, 姚敏, 王理, 钱琦, 时杨, 蔚丹丹, 陆少林, 姚登福. 下调GPC-3基因转录对肝癌细胞侵袭及血管新生的抑制作用. 世界华人消化杂志 2014; 22(16): 2221-2228
Revised: April 13, 2014
Accepted: April 17, 2014
Published online: June 8, 2014
AIM: To investigate whether down-regulation of glypican-3 (GPC-3) gene transcription by short hairpin RNAs (shRNAs) inhibits hepatoma MHCC-97H cell invasion and angiogenesis.
METHODS: Specific GPC-3 shRNAs were transfected into MHCC-97H cells. GPC-3 mRNA and protein expression was analyzed by fluorescence quantitative PCR and Western blot, respectively. Hepatoma cell proliferation was detected by 5-ethynyl-2′-deoxyuridine and sulforhodamine B assay, and cell migration and invasion were assessed by wound healing and transwell assays.
RESULTS: After MHCC-97H cells were transfected with shRNA1, GPC-3 mRNA expression was down-regulated by 75.6% (t = 15.473, P < 0.001), cell proliferation was inhibited significantly, and cell migration and invasion were decreased. β-catenin expression was down-regulated by 67.7%, and glioma-associated oncogene 1 (Gli1) expression was up-regulated by 53.5% in MHCC-97H cells. The expression of vascular endothelial growth factor (VEGF) was significantly decreased (54.2%, t = 46.746, P < 0.001) in cells transfected with shRNA1 compared with control cells.
CONCLUSION: shRNA-mediated GPC-3 knockdown inhibits the migration, invasion and angiogenesis of hepatoma cells possibly through Wnt/β-catenin and Hh signaling pathways.
- Citation: Tai BJ, Yao M, Wang L, Qian Q, Shi Y, Yu DD, Lu SL, Yao DF. Down-regulation of glypican-3 gene transcription inhibits hepatoma cell invasion and angiogenesis. Shijie Huaren Xiaohua Zazhi 2014; 22(16): 2221-2228
- URL: https://www.wjgnet.com/1009-3079/full/v22/i16/2221.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i16.2221
磷脂酰肌醇蛋白聚糖-3(glypican-3, GPC-3)在正常肝组织中未见表达, 胎肝和肝癌(hepatocellular carcinoma, HCC)组织呈过表达状态[1,2], 是HCC肝癌特异诊断最有应用前景的生物标志[3,4]. HCC进展快、复发率高和预后差, 与肝癌的血管新生、癌细胞的高侵袭性等诸多因素相关[5,6]. GPC-3蛋白锚定于细胞膜, 与Wnt/β-catenin和Hedgehogs(Hh)等信号通路密切相关[7,8]; 肝癌发生发展过程中, Wnt/β-catenin通路异常激活, β-连环蛋白积聚, 对肝癌细胞增殖和生长起促进作用; Hh通路信号增强, 激活锌指转录因子脑胶质瘤相关癌基因1(glioma-associated oncogene 1, Gli1), 在组织架构、癌细胞增殖和分化方面发挥作用[9-11]; 然而, 肝组织GPC-3过表达与肝癌细胞侵袭和血管新生的关系以及作用机制尚待阐明[12,13]. 本文以特异性shRNA沉默GPC-3基因转录, 观察对Wnt/β-catenin通路中β-catenin和Hh通路中Gli1表达的作用, 分析干预GPC-3转录对肝癌细胞迁移侵袭和血管新生的影响.
人HepG2及MHCC-97H细胞株购自中科院上海细胞库; DMEM完全培养基购自Gibco公司; GPC-3和GAPDH引物由TaKaRa公司设计合成; β-catenin和Gli1引物由Invitrogen公司合成; TRIzol试剂、LipoD293TM和GenJetTM转染试剂均购自Invitrogen公司; GPC-3购自美国Abcam公司; 基质胶购自BD公司; 血管内皮生长因子(vascular endothelial growth factor, VEGF)ELISA检测试剂盒购自上海蓝基公司.
1.2.1 质粒构建和转染: 按文献[14]合成并构建4种干扰序列和阴性对照序列. 细胞以含10%FBS DMEM完全培养基于37 ℃含5%CO2培养箱培养. 转染前24 h将融合度为70%左右细胞接种6孔板中, 于转染前1 h更换新培养基. 最有效的shRNA1(正向: 5'-CACCGCGGTTACTGCAATGTGGTCATTCAAGAGATGACCA CATTGCAGTAACCGCTTTTTTG-3', 反向: 5'-GATCCAAAAAAGCGGTTACTGCAATGTGGTCATCTCTTGAATGACCACATTGCAGTAACCGC-3')作为干扰组及阴性对照(正向: 5'-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3', 反向5'-GATCCAAAAAATTCTCCGAACGTG TCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3')质粒作为阴性对照组分别用LipoD293TM转染试剂转染至MHCC-97H细胞, 并培养未处理细胞作为空白对照. 将shRNA(沉默GPC-3有效序列)作为干扰组及阴性对照质粒作为阴性对照组分别用GenJetTM(Ver Ⅱ for HepG2细胞)转染试剂转染至HepG2细胞, 培养未处理HepG2细胞作为空白对照. 12-18 h后换液, 继续培养.
1.2.2 总RNA提取、引物及荧光定量PCR: MHCC-97H细胞分别设转染组4组, 阴性对照及空白对照(n = 3). 转染48 h后, 以TRIzol试剂提取细胞总RNA. 检测总RNA及A260/280. 引物序列(5'-3'): GPC-3(NM_004484), F-CGAGATAAG CACCTTTCACAACC和R-AGAAGAAGCACACCACCGAGA; β-catenin(NM-001904), F-AAAGCGGCTGTTAGTCACTGG和R-AAAGCGGCTGTTAGTCACTGG; Gli1(NM-005269), F-GCACCACATCAACAGTGAGC和R-GCGTCTTGAGGTTTTCAAGG; GAPDH(NM_017008), F-CAAGGTCATCCATGACAACTTTG和R-GTCCACCACCCTGTTGCTGTAG. RT-PCR扩增参数如下: 95 ℃ 30 s, 94 ℃ 5 s, 60 ℃ 45 s, 40个循环, 4 ℃保存. 以Ct比较法对GPC-3基因相对定量: 即ΔCt = Ct目的基因-Ct内参基因, 相对表达量 = 2-ΔΔCt.
1.2.3 Western blot检测蛋白浓度: 转染细胞及空白对照细胞, 于转染48 h后收集细胞, 提取蛋白后以BCA法定量. 制备10%SDS聚丙烯酰胺凝胶, 每泳道蛋白量50 μg, 加5×SDS上样缓冲液, 封口膜封口后于沸水煮8 min上样, 电泳恒压60 V×40 min, 后110 V×60 min, 转移至PVDF膜(膜在甲醇浸泡于转膜缓冲液中平衡10 min), 配制5%脱脂牛奶封闭液(脱脂奶粉2.5 g+TBS-T 50 mL)于玻璃平皿中, 室温下脱色摇床封闭1 h. 取膜用TBS-T漂洗5 min×4次. 加兔抗人GPC-3(1:250)或β-catenin(美国CST公司, 1:1000)或Gli1(美国CST公司, 1:1000), β-actin抗体(1:1000)作为内参, 4 ℃过夜, 洗涤, 辣根过氧化物酶标记的羊抗兔及羊抗鼠IgG二抗(1:1000)孵育1 h, 洗涤后ECL显色并拍照.
1.2.4 细胞增殖分析: 细胞转染48 h后, 胰蛋白酶消化细胞, 调整细胞浓度为1×104/孔, 细胞贴壁后每孔加入100 μL 50 μmol/L EdU培养基孵育2 h, PBS洗涤2遍, 加4%多聚甲醛固定液, 室温孵育30 min, 每孔加入100 μL 1×Apollo®染色反应液, 室温、避光、脱色摇床孵育30 min后加入100 μL含0.5%TritonX-100的PBS脱色摇床清洗3次, 每孔加入100 μL 1×Hoechst33342反应液, 避光、室温、脱色摇床孵育30 min, PBS漂洗3遍, 立即于倒置荧光显微镜下观察并拍照.
1.2.5 划痕试验分析细胞愈合能力: MHCC-97H细胞于转染48 h后, 当融合度为90%左右时, 用10 μL无菌移液器枪头在培养细胞的6孔板平皿中央划出一条直径为1 mm左右的直线划痕, PBS轻洗3遍, 洗去漂浮的脱落细胞, 每孔加入2 mL无血清培养基, 在倒置相差显微镜下取划痕处几个不同视野拍照(0 h), 继续于37 ℃含5%CO2培养箱中培养24 h并拍照, 观察各组细胞由划痕边缘向划痕中央迁移后剩下距离(mm, ×200), 根据划痕部位细胞愈合的相对距离判断细胞运动能力. 划痕愈合率(%) = (0 h划痕宽度-24 h划痕宽度)/0 h划痕宽度×100%.
1.2.6 Transwell法分析迁移能力: 先将Matrigel胶用无血清DMEM-H培养基1:3稀释, 每个侵袭小室加60 μL, 迁移实验不加Matrigel. 各细胞转染48 h后, 胰蛋白酶消化, PBS洗涤2遍, 用无血清培养基调整细胞浓度为1.0×106/mL, 每孔取100 μL接种于Transwell小室上室, 下室加入600 μL完全培养基, 37 ℃, 5%CO2培养箱培养24 h后检测, 迁移20 h后检测. 棉签擦去滤膜上层贴壁生长但未能穿过膜细胞, 将上室置于950 mL/L乙醇室温固定30 min, 0.1%结晶紫染色15 min后, 置显微镜下观察并拍照, 任取中央及四周5个低倍视野(200×)细胞计数(n = 3), 计算平均值; 33%醋酸脱色, 酶标仪测A570, 侵袭抑制率(%) = [(A对照-A转染)/A对照]×100%.
1.2.7 β-catenin及胰岛素样生长因子Ⅱ(insulin-like growth factor Ⅱ, IGF-Ⅱ)荧光分析: 转染48 h后, 胰酶消化细胞, 调整细胞为2×105/mL, 接种于铺有无菌盖玻片6孔板内, 培养24 h后细胞贴壁爬片, 取出盖玻片, PBS洗3遍, 用4%多聚甲醛固定30 min, 0.5%Triton-100透膜处理15 min, PBS洗3遍后用含5%BSA封闭1 h, PBS漂洗, 兔抗人β-catenin抗体(1:100)、鼠抗人IGF-Ⅱ抗体(1:100)分别于4℃过夜, PBS漂洗3遍后用Cy3标记抗兔或抗小鼠荧光二抗(1:1000)室温避光孵育1 h, PBS漂洗3遍, 添加DAPI避光染色15 min, 洗涤后滴加抗荧光淬灭封闭液封片, 于倒置荧光显微镜下观察并拍照.
1.2.8 VEGF定量: 将shRNA1质粒及阴性对照转染至细胞, 设置未处理细胞为空白组, 分别于24、48和72 h收集细胞上清液(n = 3), 按ELISA试剂盒说明操作检测VEGF含量.
统计学处理 所有数据采用SPSS17.0和GraphPad Prism 5.0统计分析软件进行分析处理及作图, 所有计量资料以mean±SD表示, 样本均数两两比较采用独立样本t检验, 多样本均数比较采用单因素方差分析(One-way ANOVA), P<0.05为差异有统计学意义.
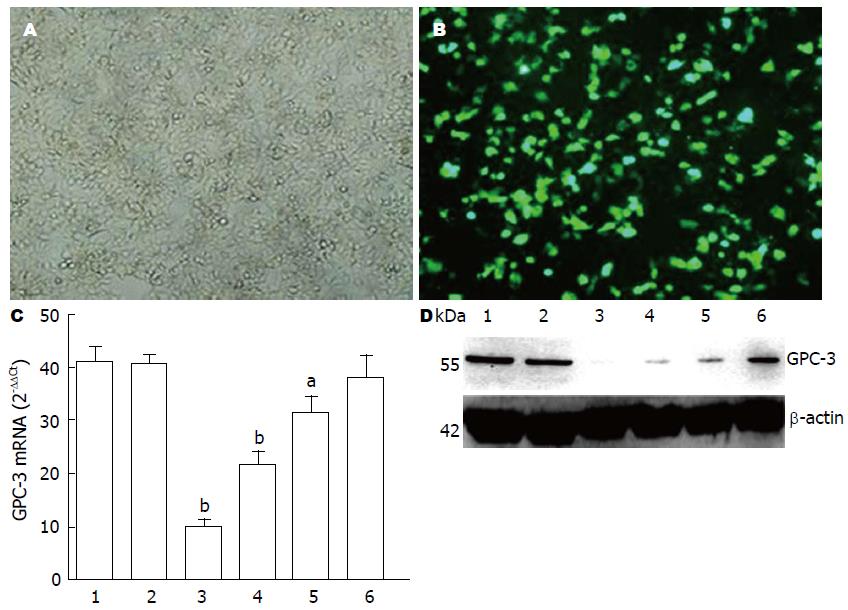
将GPC-3特异的shRNA转染人肝癌MHCC-97H细胞, 培养、荧光显微镜观察(n = 5)、拍照, 计算转染效率(图1A, B). 结果显示转染效率在80%以上, 4种shRNA转染对MHCC-97H细胞GPC-3 mRNA均有干扰, shRNA1、shRNA2、shRNA3和shRNA4分别为75.6%、47.5%、22.8%和6.7%, 其中shRNA1对MHCC-97H细胞GPC-3 mRNA转录沉默效果最佳(图1C), (t =15.473, P<0.001); 在MHCC-97H细胞GPC-3蛋白水平上, Western blot分析显示以shRNA1明显抑制GPC-3蛋白表达, 干扰效果最好(图1D).
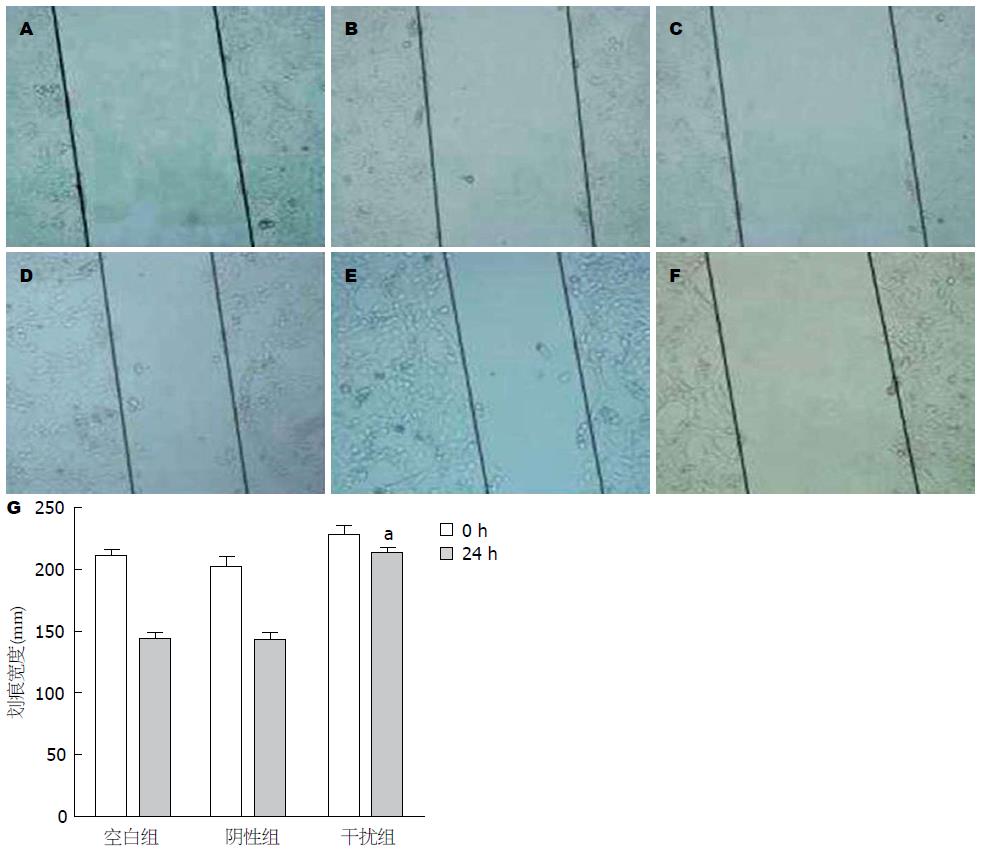
干预MHCC-97H细胞GPC-3转录后, 空白组、对照组和干扰组划痕实际平均宽度(n = 3), 0 h分别为210.7、201.3和228.3 mm, 24 h分别为142.7、141.6和213.6 mm, 划痕愈合率(图2A)分别为32.2%、27.4%和6.4%, 干扰组MHCC-97H细胞的划痕愈合速度明显慢于空白组(t = 32.697, P<0.001), (图2B); 以Transwell法分析MHCC-97H细胞侵袭能力, 沉默GPC-3表达后显示肝癌细胞侵袭力明显受抑. 与空白组相比, MHCC-9H细胞侵袭抑制率为69.1%(t = 39.647, P<0.001).
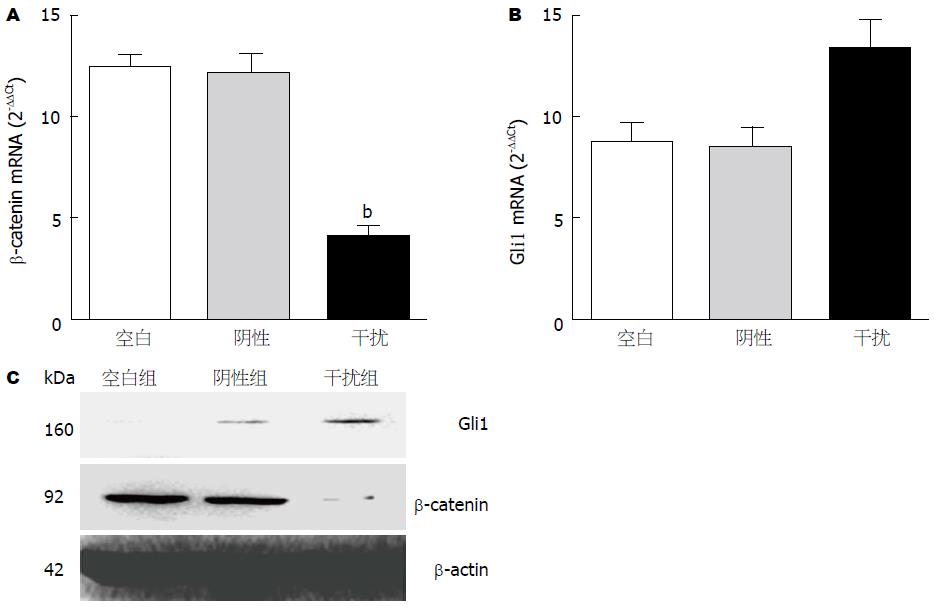
转染GPC-3-shRNA1及阴性对照质粒于HCC细胞, 检测β-catenin及Gli1mRNA水平, 显示下调β-catenin mRNA(图3A)并上调Gli1 mRNA表达(图3B); 与空白组相比, MHCC97-H细胞中β-catenin mRNA抑制67.7%(t = 18.4, P<0.001); Gli1 mRNA上调53.3%(t = -4.824, P = 0.008); Western blot分析显示β-catenin明显下调, Gli1明显上调(图3C).
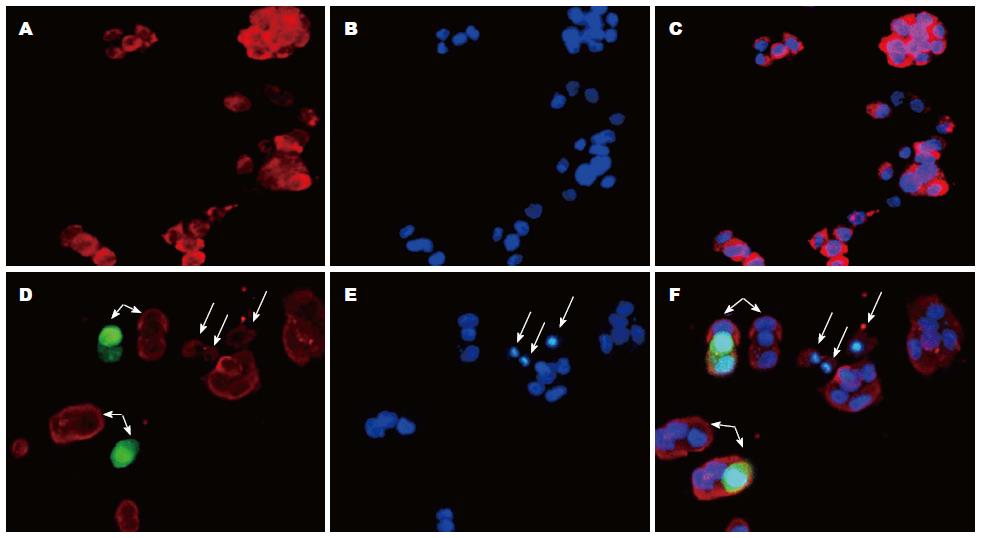
免疫荧光显示IGF-Ⅱ广泛存在于MHCC-97H细胞膜、细胞浆和细胞核; shRNA1转染沉默MHCC-97H细胞中GPC-3表达后, 细胞中IGF-Ⅱ表达明显下调, MHCC-97H细胞凋亡明显增加(图4).
ELISA法检测MHCC-97H细胞中VEGF表达, 显示下调MHCC-97H细胞中GPC-3的表达后VEGF明显下调且呈时间依赖性. MHCC-97H细胞干扰组24、48和72 h与MHCC-97H细胞空白组相比, VEGF表达分别下降36.8%(t24 = 14.543, P<0.001)、46.2%(t48 = 55.212, P<0.001)和54.2%(t72 = 46.746, P<0.001).
位于X染色体(Xq26)的GPC-3, 其核心蛋白由40 kDa的氨基末端亚基和30 kDa的羧基末端亚基组成, 通过糖基磷脂酰肌醇锚定于细胞膜表面[15,16]. 羧基末端接近细胞膜的区域含有两条硫酸类肝素聚糖链. 葡糖氨基聚糖参与调节多条信号通路, 与多种生长因子如Wnts、Hh、胰岛素样生长因子Ⅱ(insulin-like growth factor Ⅱ, IGF-Ⅱ)、转化生长因子-β、成纤维生长因子及骨形态生成蛋白等结合并介导HCC发生发展[17,18]; 通过调节基质金属蛋白酶和尿激酶型纤溶酶原激活物水解细胞外基质、促进肿瘤侵袭与转移[19,20]. 本文构建高效特异的GPC-3-shRNA干扰质粒, 转染具有高转移潜能肝癌MHCC-97H细胞观察下调GPC-3基因转录对肝癌细胞增殖和血管形成的影响、分析对肝癌相关Wnt/β-catenin通路中关键信号分子的表达与改变特征.
GPC-3以调节自分泌/旁分泌的经典Wnt信号通路刺激HCC细胞生长, 其过表达可使细胞结合Wnt能力增强. Wnts与HS链结合形成磷脂酰蛋白聚糖-Wnt/生长因子复合物, 在硫酸酯酶-2(sulfatase 2, SULF-2)作用下Wnts从HS链上释放, 与Frizzled受体结合进而激活Wnt/Frizzled下游区域的信号转导, 促进HCC细胞增殖[21,22]. shRNA有效干预GPC-3基因转录, 显示shRNA1可显著抑制细胞增殖、迁移和侵袭, 可影响肝癌相关Wnt/β-catenin和Hh通路. β-catenin是Wnt/β-catenin信号通路的关键分子, Wnt信号激活将致β-catenin在胞内大量积聚, 进入胞核触发肝癌发生等. shRNA1转染MHCC-97H细胞后, 在mRNA水平上β-catenin下调67.7%; 但Gli1分别上调53.3%. 沉默GPC-3可下调β-catenin并上调Gli1表达; 推测GPC-3可经上调β-catenin激活Wnt/β-catenin通路[23,24], 与Hh竞争结合Ptch受体下调Gli1表达而抑制Hh通路[9,25], 促进肝癌发展.
下调GPC-3基因转录影响肝癌相关信号通路, 是否影响新血管生成, 本文观察了对IGF-Ⅱ和VEGF的表达. 肝脏是合成和分泌IGF-Ⅱ主要器官, 癌变过程中激活启动子P3产生胚胎表型逆转, 使IGF-Ⅱ过表达. IGF-Ⅱ调节胚胎生长发育、刺激DNA合成、促进细胞有丝分裂, 在肝癌转移中起重要作用[26,27]. 下调MHCC-97H细胞GPC-3表达, IGF-Ⅱ表达显著下调, 并诱导细胞凋亡; VEGF为多功能细胞因子, 具有促内皮细胞分裂、增殖和血管构建, 细胞迁移, 诱导蛋白水解酶、间质胶原酶和组织因子表达. 癌组织VEGF过表达, 启动新生血管形成是肝癌生长、侵袭和转移基础, 为癌细胞播散途径[28,29]. 下调GPC-3明显抑制VEGF表达, 且呈时间依赖性, 显示靶向GPC-3可潜在抑制肝癌细胞转移发生.
肝癌是我国常见的恶性肿瘤, 为典型多血管肿瘤, 肝细胞癌变机制复杂, 多种信号通路参与. 新生血管形成同样是肝癌细胞增殖和转移的重要条件, 血管生长因子经与特异性受体结合, 直接刺激内皮细胞增殖, 并增加血管通透性, 不仅促进新生血管形成, 且有利于脱落的肝癌细胞进入血管或向肝外转移. 肝癌GPC-3高表达, 为有助于早期诊断特异标志[30]; 癌胚型GPC-3激活促进肝细胞恶性转化与肝癌的转移发生[31], 新近证实GPC-3基因有可能成为HCC靶向治疗的潜在分子靶目标, Wnt/β-catenin信号通路与HCC发展密切相关, 调控肝细胞生长、分化、增殖和凋亡, 也为癌症的诊断和治疗带来契机.
肝癌是我国常见的恶性肿瘤, 他的早期诊断与转移监测是全球医学界的难题. 胎肝和肝癌组织中磷脂酰肌醇蛋白多糖-3(glypican-3, GPC-3)过表达, GPC-3分析已是肝癌特异诊断的生物标志, 有望成为抑制肝癌生长与转移的靶目标.
高润平, 教授, 吉林大学第一医院肝胆胰内科
癌胚型GPC-3激活促进肝细胞恶性转化与肝癌的转移发生, 新近证实GPC-3表达与Wnt/β-catenin信号通路与肝癌发展密切相关, 调控肝细胞生长、分化、增殖和凋亡, 可为肝癌基因治疗带来新契机.
Yao等报道人肝癌组织GPC-3阳性率为80.6%, 远癌组织未见阳性; 肝癌患者外周血GPC-3 mRNA阳性率为70.7%, 肝癌伴远处转移患者阳性为100%, 提示GPC-3及基因转录与肝癌密切相关, 是否具有基因治疗价值有待证实.
本研究构建靶向肝癌GPC-3的高效、特异的shRNA干扰质粒, 在体外, 成功转染具高转移潜能MHCC-97H细胞, 下调GPC-3基因转录明显抑制肝癌细胞增殖, 证实其机制为抑制Wnt/β-catenin通路关键信号分子发挥作用.
在体外以高效、特异的shRNA干扰肝癌GPC-3转录, 明显抑制肝癌细胞增殖, 显示在肝癌过表达的GPC-3为新发现治疗靶点, 需更多的肝癌发生或动态体内模型深入研究, 论证干预GPC-3基因转录有助于肝癌的靶向治疗.
作者构建与筛选出对肝癌GPC-3干预具有的特异性shRNA, 转染肝癌MHCC-97H细胞, 显著抑制肝癌细胞迁移和侵袭作用.
编辑: 郭鹏 电编:鲁亚静
| 1. | Qiao SS, Cui ZQ, Gong L, Han H, Chen PC, Guo LM, Yu X, Wei YH, Ha SA, Kim JW. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1718-1724. [PubMed] |
| 2. | Yao M, Yao DF, Bian YZ, Zhang CG, Qiu LW, Wu W, Sai WL, Yang JL, Zhang HJ. Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:289-294. [PubMed] [DOI] |
| 3. | Liu XF, Hu ZD, Liu XC, Cao Y, Ding CM, Hu CJ. Diagnostic accuracy of serum glypican-3 for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Biochem. 2014;47:196-200. [PubMed] [DOI] |
| 4. | Zaakook M, Ayoub M, Sinna EA, El-Sheikh S. Role of glypican-3 immunocytochemistry in differentiating hepatocellular carcinoma from metastatic carcinoma of the liver utilizing fine needle aspiration cytology. J Egypt Natl Canc Inst. 2013;25:173-180. [PubMed] [DOI] |
| 5. | Cheng J, Wang W, Zhang Y, Liu X, Li M, Wu Z, Liu Z, Lv Y, Wang B. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: systematic review and meta-analysis. PLoS One. 2014;9:e87011. [PubMed] [DOI] |
| 6. | Kennedy AS, Sangro B. Nonsurgical treatment for localized hepatocellular carcinoma. Curr Oncol Rep. 2014;16:373. [PubMed] |
| 7. | Gao W, Ho M. The role of glypican-3 in regulating Wnt in hepatocellular carcinomas. Cancer Rep. 2011;1:14-19. [PubMed] |
| 8. | Zheng X, Zeng W, Gai X, Xu Q, Li C, Liang Z, Tuo H, Liu Q. Role of the Hedgehog pathway in hepatocellular carcinoma (review). Oncol Rep. 2013;30:2020-2026. [PubMed] |
| 9. | Lu JT, Zhao WD, He W, Wei W. Hedgehog signaling pathway mediates invasion and metastasis of hepatocellular carcinoma via ERK pathway. Acta Pharmacol Sin. 2012;33:691-700. [PubMed] [DOI] |
| 10. | Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn WK. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. [PubMed] [DOI] |
| 11. | Allan BJ, Wang B, Davis JS, Parikh PP, Perez EA, Neville HL, Sola JE. A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg. 2014;49:166-71; discussion 171. [PubMed] [DOI] |
| 12. | Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat. 2014;21:153-162. [PubMed] |
| 13. | Higgins MC, Soulen MC. Combining locoregional therapies in the treatment of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30:74-81. [PubMed] |
| 14. | Yu D, Dong Z, Yao M, Wu W, Yan M, Yan X, Qiu L, Chen J, Sai W, Yao D. Targeted glypican-3 gene transcription inhibited the proliferation of human hepatoma cells by specific short hairpin RNA. Tumour Biol. 2013;34:661-668. [PubMed] [DOI] |
| 15. | Enan ET, El-Hawary AK, El-Tantawy DA, Abu-Hashim MM, Helal NM. Diagnostic role of glypican 3 and CD34 for differentiating hepatocellular carcinoma from nonmalignant hepatocellular lesions. Ann Diagn Pathol. 2013;17:490-493. [PubMed] [DOI] |
| 16. | Witjes CD, ten Kate FJ, Verhoef C, de Man RA, IJzermans JN. Immunohistochemical characteristics of hepatocellular carcinoma in non-cirrhotic livers. J Clin Pathol. 2013;66:687-691. [PubMed] [DOI] |
| 17. | Yao M, Yao DF, Bian YZ, Wu W, Yan XD, Yu DD, Qiu LW, Yang JL, Zhang HJ, Sai WL. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:171-179. [PubMed] [DOI] |
| 18. | Lagana SM, Salomao M, Bao F, Moreira RK, Lefkowitch JH, Remotti HE. Utility of an immunohistochemical panel consisting of glypican-3, heat-shock protein-70, and glutamine synthetase in the distinction of low-grade hepatocellular carcinoma from hepatocellular adenoma. Appl Immunohistochem Mol Morphol. 2013;21:170-176. [PubMed] |
| 19. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [PubMed] [DOI] |
| 20. | Daniele A, Divella R, Quaranta M, Mattioli V, Casamassima P, Paradiso A, Garrisi VM, Gadaleta CD, Gadaleta-Caldarola G, Savino E. Clinical and prognostic role of circulating MMP-2 and its inhibitor TIMP-2 in HCC patients prior to and after trans-hepatic arterial chemo-embolization. Clin Biochem. 2014;47:184-190. [PubMed] [DOI] |
| 21. | Marquardt JU, Quasdorff M, Varnholt H, Curth HM, Mesghenna S, Protzer U, Goeser T, Nierhoff D. Neighbor of Punc E11, a novel oncofetal marker for hepatocellular carcinoma. Int J Cancer. 2011;128:2353-2363. [PubMed] [DOI] |
| 22. | O'Beirne J, Farzaneh F, Harrison PM. Generation of functional CD8+ T cells by human dendritic cells expressing glypican-3 epitopes. J Exp Clin Cancer Res. 2010;29:48. [PubMed] [DOI] |
| 23. | Gu DL, Chen YH, Shih JH, Lin CH, Jou YS, Chen CF. Target genes discovery through copy number alteration analysis in human hepatocellular carcinoma. World J Gastroenterol. 2013;19:8873-8879. [PubMed] [DOI] |
| 24. | Liu J, Ruan B, You N, Huang Q, Liu W, Dang Z, Xu W, Zhou T, Ji R, Cao Y. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the β-catenin pathway in hepatic oval cells. PLoS One. 2013;8:e79409. [PubMed] [DOI] |
| 25. | Arzumanyan A, Sambandam V, Clayton MM, Choi SS, Xie G, Diehl AM, Yu DY, Feitelson MA. Hedgehog signaling blockade delays hepatocarcinogenesis induced by hepatitis B virus X protein. Cancer Res. 2012;72:5912-5920. [PubMed] [DOI] |
| 26. | Pan Z, Chen C, Long H, Lei C, Tang G, Li L, Feng J, Chen F. Overexpression of GPC3 inhibits hepatocellular carcinoma cell proliferation and invasion through induction of apoptosis. Mol Med Rep. 2013;7:969-974. [PubMed] |
| 27. | Kessler SM, Pokorny J, Zimmer V, Laggai S, Lammert F, Bohle RM, Kiemer AK. IGF2 mRNA binding protein p62/IMP2-2 in hepatocellular carcinoma: antiapoptotic action is independent of IGF2/PI3K signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G328-G336. [PubMed] [DOI] |
| 28. | Wang D, Luo L, Chen W, Chen LZ, Zeng WT, Li W, Huang XH. Significance of the vascular endothelial growth factor and the macrophage migration inhibitory factor in the progression of hepatocellular carcinoma. Oncol Rep. 2014;31:1199-1204. [PubMed] |
| 29. | Moeini A, Cornellà H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1:83-93. [PubMed] |
| 30. | Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39:410-433. [PubMed] [DOI] |
| 31. | Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and survivin in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in liver fine-needle aspiration biopsies. Diagn Cytopathol. 2009;37:629-635. [PubMed] [DOI] |