修回日期: 2013-11-08
接受日期: 2013-11-20
在线出版日期: 2014-01-08
目的: 探讨结肠癌术后患者切除修复交叉互补基因1(exsicion repair cross-complementing group 1, ERCC1)、胸苷酸合成酶(thymidylate synthase, TS)表达与术后辅助化疗预后的关系.
方法: 选取76例初次诊断为结肠癌、行结肠癌根治术、术后病理诊断为Ⅱ、Ⅲ期的患者; 所有患者术后均采用FOLFOX4(L-OHP+5-Fu+CF)辅助化疗方案. 并进行3年以上随访. 运用免疫组织化学方法检测患者癌组织中核苷酸ERCCl、TS蛋白表达. 回顾性分析结肠癌患者组织中ERCC1、TS表达与术后辅助化疗预后的关系.
结果: 76例结肠癌患者组织中ERCC1和TS的阳性表达率分别为36.8%和43.4%. ERCC1和TS阳性表达率与患者肿瘤的分化程度存在负相关. P值分别为0.019和0.024. ERCC1阴性表达者中位无病生存期(33.08 mo)明显长于阳性表达者(27.86 mo), 两组比较差异有统计学意义(P<0.05). TS阴性表达者中位无病生存期(32.74 mo)明显长于阳性表达者(29.09 mo), 两组比较差异有统计学意义(P<0.05). 多因素Cox回归分析表明, ERCC1、TS阳性患者术后辅助化疗预后差(HR = 3.50, 95%CI: 1.59-7.73, P = 0.002; HR = 0.52, 95%CI: 0.35-0.87, P = 0.010).
结论: ERCC1与TS表达水平可以作为预测结肠癌术后辅助化疗预测的分子标志物.
核心提示: 分析结肠癌患者组织中切除修复交叉互补基因1(exsicion repair cross-complementing group 1, ERCC1)、胸苷酸合成酶(thymidylate synthase, TS)表达与FOLFOX4术后辅助化疗预后的关系. ERCC1阴性表达者中位生存期(33.08 mo)明显长于阳性表达者(27.86 mo). TS阴性表达者中位生存期(32.74 mo)明显长于阳性表达者(29.09 mo), 比较差异均有统计学意义(P<0.05). 多因素Cox回归分析表明, ERCC1、TS阳性患者术后辅助化疗预后差.
引文著录: 王维民, 邓建良, 顾贤成, 汤月华, 张国强, 周炎. ERCC1、TS在结肠癌组织中的表达及与术后辅助化疗预后的关系. 世界华人消化杂志 2014; 22(1): 24-30
Revised: November 8, 2013
Accepted: November 20, 2013
Published online: January 8, 2014
AIM: To explore the relationship between excision repair cross-complementing group 1 (ERCC1) expression, thymidylate synthase (TS) expression and the prognosis in colon cancer after postoperative adjuvant chemotherapy.
METHODS: Seventy-six patients who were diagnosed with colon cancer for the first time were enrolled in our study. All the patients received radical operation, were pathologically diagnosed with stage Ⅱ or Ⅲ disease, accepted FOLFOX4 (L-OHP+5-Fu+CF) chemotherapy and were followed at least 3 years. Immunohistochemistry was used to detect ERCC1 and TS expression levels in colon cancer. The relationship between the expression of ERCC1 and TS and postoperative survival was analyzed retrospectively.
RESULTS: The positive expression rates of ERCC1 and TS in colon cancer were 36.8% and 43.4%, respectively. There were a negative correlation between the positive expression of ERCC1 and TS and tumor differentiation (P = 0.019 and 0.024). The median survival time was significantly longer in patients with negative ERCC1 expression than in those with positive expression (P < 0.05), and in patients with negative TS expression than in those with positive expression (P < 0.05). Cox multivariate regression analysis revealed that patients with positive ERCC1 and TS expression did not benefit from chemotherapy (HR = 3.50, 95%CI: 1.59-7.73, P = 0.002; HR = 0.52, 95%CI: 0.35-0.87, P = 0.010).
CONCLUSION: ERCC1 and TS may be biomarkers for predicting the prognosis of colon cancer patients receiving chemotherapy.
- Citation: Wang WM, Deng JL, Gu XC, Tang YH, Zhang GQ, Zhou Y. ERCC1 and TS expression and prognosis in colon cancer after postoperative adjuvant chemotherapy. Shijie Huaren Xiaohua Zazhi 2014; 22(1): 24-30
- URL: https://www.wjgnet.com/1009-3079/full/v22/i1/24.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v22.i1.24
结肠癌是最常见的消化系肿瘤之一[1]. 其发病情况有显著的地区性差异. 高发区主要集中在北美等发达国家[2,3]. 我国尚处于低发区[4,5]. 但是近几年来, 随着经济的发展. 人们的生活方式尤其是饮食结构的变化. 结肠癌已经成为我国发病率上升最快的恶性肿瘤之一. 严重威胁着国人的生命和健康[6-8].
结肠癌的治疗上首选根治性手术[9]. 但因其存在高转移、高复发的风险. 故术后辅助化疗在肠癌的综合治疗中起至关重要作用[10-12]. 根据NCCN指南, 奥沙利铂(L-OHP)与氟脲嘧啶类联合的FOLFOX6(L-OHP+5-Fu+CF)方案已成为结肠癌术后的标准化疗方案. 其疗效亦得到肯定. 但仍存在相当一部分患者术后易复发或转移[13-15]. 为了能更有效的提高术后辅助化疗疗效. 我们通过对76例结肠癌术后患者的组织标本中核苷酸切除修复交叉互补基因1(exsicion repair cross-complementing group 1, ERCC1)、胸苷酸合成酶(thymidylate synthase, TS)蛋白表达的检测. 分析他们与临床病理特征的关系. 并探讨分子标志物联合检测在预测结肠癌术后辅助化疗疗效及预后中的作用. 为结肠癌的个体化治疗提供临床依据.
76例标本取自江苏大学附属宜兴医院2006-01/2009-12手术治疗的结肠癌患者. 均经病理证实为结肠癌患者. 其中男45例, 女31例, 年龄31-72岁. 中位年龄52岁. 按1997年国际抗癌联盟(Union for International Cancer Control, UICC)TNM标准进行分期: Ⅱ期36例, Ⅲ期40例. 术后患者均应用FOLFOX4方案(奥沙利铂130 mg/m2, 静脉滴注d1; 亚叶酸钙200 mg/m2, 静脉滴注d1-d2; 5-氟尿嘧啶400 mg/m2, 静脉推注d1-d2; 5-氟尿嘧啶600 mg/m2, 静脉持续滴注d1-d2). 每2周重复, 至少完成6个周期的化疗. 化疗后通过上门或电话的方式进行随访. 直至患者死亡. 若末次随访仍然存活则定为截尾值. 随访截止时间2012-12. 鼠抗人ERCC1、TS单克隆抗体购自Santa Cruz生物工程公司. 二步法免疫组织化学试剂盒购自北京中杉金桥生物技术有限公司. 石蜡切片机及漂片处理仪、图文分析系统为日本莱卡公司产品.
1.2.1 实验: 标本用4%甲醛固定, 石蜡包埋, 常规切片, 厚度4 μm. 采用二步法免疫组织化学检测结肠癌组织ERCCl、TS蛋白的表达. 常规脱蜡、水化后进行预处理. 3%H2O2室温培育10 min. 蒸馏水洗3次. 每次2 min. 分别滴加1:100稀释的抗ERCCl、TS单克隆抗体. 室温培育20 min, DAB显色, 苏木精对比染色、脱水, 二甲苯透明, 中性树脂封固, 显微镜下观察.
1.2.2 免疫组织化学结果判断标准: ERCC1、TS蛋白阳性染色者均在细胞核和/或细胞浆呈棕黄色沉着. 在光镜下(400倍视野)计算1000个肿瘤细胞中阳性细胞数. 阴性: 不表达或阳性细胞数<10%; 阳性: 视野中阳性细胞数≥10%. 病理结果由2位病理科医生在无法获知临床资料及预后的情况下独立阅片判定.
统计学处理 采用STATA10.0统计软件进行统计学分析. 定性资料比较采用Fisher确切概率法. 总体生存分析采用Kaplan-Meier生存曲线表示. 生存率的比较采用Log-rank检验. 影响因素分析采用Cox多因素回归模型. 检验水准α = 0.05双侧, P<0.05为差异有统计学意义.
76例结肠癌患者中, ERCCl蛋白阳性28例(36.8%). 阴性48例(63.2%); TS蛋白阳性33例(43.4%). 阴性43例(56.6%). ERCCl、TS蛋白表达与患者临床分期、年龄、性别均无明显相关性(P>0.05, 表1, 表2). 但两者表达均随着肿瘤的分化程度降低而明显增高(P<0.05).
| 临床病理特征 | n | ERCC1 | P值 | |
| 阴性表达 | 阳性表达 | |||
| 性别 | 0.235 | |||
| 男 | 45 | 31(68.89) | 14(31.11) | |
| 女 | 31 | 17(54.84) | 14(45.16) | |
| 年龄(岁) | 0.348 | |||
| ≥52 | 41 | 28(68.29) | 13(31.71) | |
| <52 | 35 | 20(57.14) | 15(42.86) | |
| 病理类型 | 1.000 | |||
| 管状腺癌 | 40 | 25(62.5) | 15(37.50) | |
| 粘液腺癌/印戒细胞癌 | 15 | 10(66.67) | 5(33.33) | |
| 乳头状腺癌 | 21 | 13(61.90) | 8(38.10) | |
| 分化程度 | 0.019 | |||
| 高分化 | 23 | 5(21.8) | 18(78.3) | |
| 中分化 | 19 | 8(42.1) | 11(57.9) | |
| 低分化 | 34 | 20(58.8) | 14(41.2) | |
| 浸润程度 | 1.000 | |||
| T1+T2 | 17 | 11(64.71) | 6(35.29) | |
| T3+T4 | 59 | 37(62.71) | 22(37.29) | |
| 淋巴结转移 | 0.887 | |||
| N0 | 26 | 17(65.38) | 9(34.62) | |
| N1 | 30 | 19(63.33) | 11(36.67) | |
| N2 | 20 | 14(70.00) | 6(30.00) | |
| TNM分期 | 0.808 | |||
| Ⅱ期 | 26 | 17(65.38) | 9(34.62) | |
| Ⅲ期 | 50 | 31(62.00) | 19(38.00) | |
| 临床病理特征 | n | TS | P值 | |
| 阴性表达 | 阳性表达 | |||
| 性别 | 0.250 | |||
| 男 | 45 | 28(62.22) | 17(37.78) | |
| 女 | 31 | 15(48.39) | 16(51.61) | |
| 年龄(岁) | 1.000 | |||
| ≥52 | 41 | 23(56.10) | 18(43.90) | |
| <52 | 35 | 20(57.14) | 15(42.86) | |
| 病理类型 | 0.266 | |||
| 管状腺癌 | 40 | 20(50.00) | 20(50.00) | |
| 粘液腺癌/印戒细胞癌 | 15 | 8(53.33) | 7(46.67) | |
| 乳头状腺癌 | 21 | 15(71.43) | 6(28.57) | |
| 分化程度 | 0.024 | |||
| 高分化 | 23 | 4(17.4) | 19(82.6) | |
| 中分化 | 19 | 6(31.6) | 13(68.4) | |
| 低分化 | 34 | 18(52.9) | 16(47.1) | |
| 浸润程度 | 0.786 | |||
| T1+T2 | 17 | 9(52.94) | 8(47.06) | |
| T3+T4 | 59 | 34(57.63) | 24(42.37) | |
| 淋巴结转移 | 0.940 | |||
| N0 | 26 | 15(57.69) | 11(42.31) | |
| N1 | 30 | 18(60.00) | 12(40.00) | |
| N2 | 20 | 11(55.00) | 9(45.00) | |
| TNM分期 | 0.841 | |||
| Ⅱ期 | 26 | 15(57.69) | 11(42.31) | |
| Ⅲ期 | 50 | 36(60.00) | 24(40.00) | |
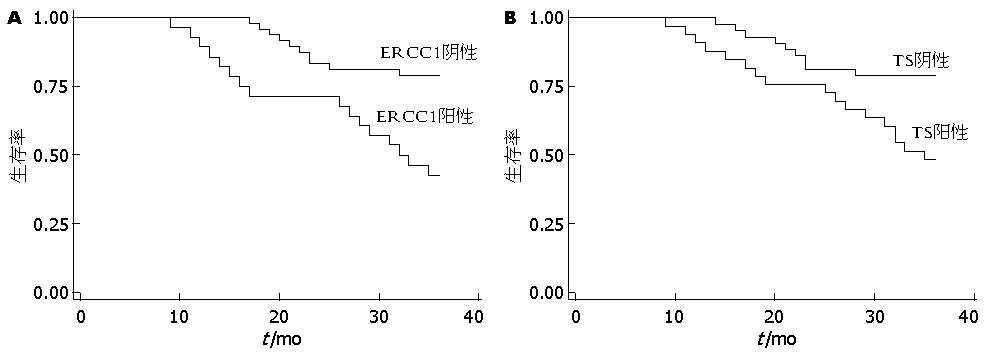
ERCC1阴性表达者中位无病生存期(median disease-free survival, DFS)为33.08 mo(95%CI: 31.39-34.78). 而ERCCl阳性表达者DFS为27.86 mo(95%CI: 24.26-31.45). 两组间的差异有统计学意义. Kaplan-Meier生存曲线显示: ERCC1阴性表达者3年生存期优于阳性表达者, 差异有统计学意义(P = 0.0009<0.01, 图1A). 多因素Cox回归模型分析表明, ERCC1阳性肠癌患者预后较差(HR = 3.50, 95%CI: 1.59-7.73). 且分化程度越高的患者, 预后越好(HR = 0.42, 95%CI: 0.23-0.76)(表3).
| 变量 | ERCC1 | TS | ||
| HR(95%CI) | P值 | HR(95%CI) | P值 | |
| 年龄(≤52 vs >52) | 0.90(0.41-1.94) | 0.780 | 0.99(0.46-2.15) | 0.987 |
| 性别(男 vs 女) | 1.68(0.74-3.80) | 0.216 | 1.59(0.71-3.60) | 0.263 |
| 浸润深度(T1/T2 vs T3/T4) | 1.40(0.59-3.32) | 0.451 | 1.42(0.60-3.38) | 0.431 |
| 淋巴结转移(N0 vs N1/N2/N3) | 1.05(0.47-2.33) | 0.906 | 1.37(0.58-3.27) | 0.476 |
| TNM分期(III vs II) | 2.10(0.93-4.77) | 0.076 | 1.34(0.59-3.03) | 0.479 |
| 组织类型 | 0.81(0.46-1.43) | 0.475 | 0.94(0.52-1.69) | 0.835 |
| 分化程度 | 0.42(0.23-0.76) | 0.004 | 0.52(0.35-0.87) | 0.032 |
| ERCC1(阳性 vs 阴性) | 3.50(1.59-7.73) | 0.002 | 2.89(1.29-6.50) | 0.010 |
TS阴性表达者中位无病生存期(DFS)为32.74 mo(95%CI: 30.77-34.71). 而TS阳性表达者DFS为29.09 mo(95%CI: 25.99-32.19), 两组间的差异有统计学意义. Kaplan-Meier生存曲线显示: TS蛋白阴性表达者3年生存期优于阳性表达者, 差异有统计学意义(P = 0.0070<0.01, 图1B). 多因素Cox回归模型分析表明, TS阳性肠癌患者预后较差(HR = 2.89, 95%CI: 1.29-6.50 ). 且分化程度越高的患者, 预后越好(HR = 0.52, 95%CI: 0.35-0.87)(表3).
ERCC1定位于19号染色体上, 是核苷酸切除修复(nucleotidc excision repair, NER)活性的标志性基因[16,17]. NER是修复DNA损伤的最主要途径, 也是修复草酸铂所致的DNA伤的最主要途径. ERCC1与XPF/ERCC4形成异二聚体而发挥功能, 参与DNA链的切割和损伤识别, 能修复紫外线、多种化学物质所造成的损伤. 是细胞存活必须的DNA修复基因[17,18].
已有临床试验证实ERCC1在细胞水平和临床上, 是一个好的奥沙利铂敏感性的标志物[19]. Kim等[20]通过免疫组织化学法检测64例进展期胃癌患者组织中ERCC1、TS和GSTP1的表达, 发现ERCCl阳性对FOLFOX方案化疗的敏感性显著低于阴性患者(P = 0.045). ERCC1是标志肿瘤患者预后和铂类化疗的重要指标, 在肿瘤治疗中具有重要作用[21,22].
TS是胸苷酸合成的限速酶, 他的功能是催化尿嘧啶脱氧核苷(dUMP)甲基化为胸腺嘧啶脱氧核苷(dTMP). 在dTMP生成过程中, TS先与dUMP及5,10-甲酰四氢叶酸(CH2FH4)形成三联复合物, 然后通过甲基化、解离、释放TS、dTMP及FH2等步骤, 最终合成DNA. 5-FU进入体内被活化成氟尿嘧啶脱氧核苷酸(FdUMP), FdUMP替代dUMP与TS及CH2FH4形成之三联复合物不易解离, 则抑制TS, 不能合成dTMP, 也就不能合成肿瘤细胞DNA, 从而发挥抗肿瘤作用[22]. TS蛋白过表达将使FdUMP不能完全抑制TS的作用, 诱发肿瘤细胞对5-FU产生耐药[23-26], 因此, TS是5-FU化疗敏感性的标志基因[23,27,28].
Yeh等[29]发现胃癌TS表达水平与5-Fu的疗效呈负相关. Temmink等[30]对几种对5-Fu继发性耐药的人类H630结肠癌细胞株H630-RI、H630-R10和H630-R与其父系H630细胞株进行对比研究, 发现各耐药细胞株对5-FU的耐药性明显增加, 细胞中TS水平也明显增加, 不但说明TS水平高的肿瘤对5-FU的敏感性差, 而且说明细胞持续暴露于5-FU也可以使细胞内TS基因突变导致基因扩增及蛋白的过度表达, 从而引起对5-Fu的继发性耐药.
我们课题组应用免疫组织化学技术检测结肠癌术后患者的ERCCl、TS蛋白表达水平. 结果显示: ERCC1蛋白在结肠癌组织中的阳性表达率高达36.8%, TS蛋白在结肠癌组织中的阳性表达率高达43.4%, 两者随肿瘤的分化程度降低而明显增高(P<0.05). ERCC1、TS蛋白低表达较高表达的结肠癌术后患者应用FOLFOX4方案化疗可获得生存受益(P<0.01).
本研究初步表明, ERCC1蛋白表达可以预测结肠癌患者对铂类化疗的敏感性; TS蛋白表达可以预测5-FU化疗药物的敏感性; 两者联合运用可以预测FOLFOX4方案的有效率, 可为结肠癌术后患者的个体化治疗提供临床理论依据.
结肠癌的治疗上首选手术. 但因其存在高转移、高复发的风险. 故术后辅助化疗起至关重要作用. 通过对76例结肠癌组织标本中ERCCl、TS蛋白表达的检测, 探讨分子标志物联合检测在预测结肠癌术后辅助化疗疗效及预后中作用.
白雪, 副主任医师, 中国人民解放军北京军区总医院普通外科
探讨结肠癌术后患者ERCC1、TS表达与术后辅助化疗预后的关系.
陈建等研究发现ERCC1-C8092A位点为A/A或A/C型, TS-5'UTR位点为2R2R、2R3C或3C3C型的晚期食管癌患者对顺铂联合5-氟尿嘧啶治疗方案更为敏感.
首次提出ERCC1、TS表达水平与结肠癌术后化疗方案-FOLFOX4疗效的关系, 提高结肠癌术后化疗的有效率, 延长其总生存期.
ERCC1与TS表达水平可以作为预测结肠癌术后辅助化疗预测的分子标志物.
文章的内容对结肠癌术后个体化化疗方案选择提供了充足的有意义的信息. 研究具有一定的新颖性.
编辑: 郭鹏 电编:鲁亚静
| 1. | Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739-750. [PubMed] [DOI] |
| 2. | Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688-1694. [PubMed] [DOI] |
| 3. | Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741-1750. [PubMed] [DOI] |
| 4. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] |
| 5. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [PubMed] [DOI] |
| 6. | Xiong F, Wu C, Bi X, Yu D, Huang L, Xu J, Zhang T, Zhai K, Chang J, Tan W. Risk of genome-wide association study-identified genetic variants for colorectal cancer in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2010;19:1855-1861. [PubMed] [DOI] |
| 7. | Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, Liu JH, Lou QY, Gan AH. Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol. 2010;16:960-965. [PubMed] |
| 8. | Yang G, Zheng W, Xiang YB, Gao J, Li HL, Zhang X, Gao YT, Shu XO. Green tea consumption and colorectal cancer risk: a report from the Shanghai Men's Health Study. Carcinogenesis. 2011;32:1684-1688. [PubMed] [DOI] |
| 9. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [PubMed] [DOI] |
| 10. | Lombardi L, Morelli F, Cinieri S, Santini D, Silvestris N, Fazio N, Orlando L, Tonini G, Colucci G, Maiello E. Adjuvant colon cancer chemotherapy: where we are and where we'll go. Cancer Treat Rev. 2010;36 Suppl 3:S34-S41. [PubMed] [DOI] |
| 11. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] |
| 12. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [PubMed] [DOI] |
| 13. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [PubMed] [DOI] |
| 14. | Bertolini F, Malavasi N, Scarabelli L, Fiocchi F, Bagni B, Del Giovane C, Colucci G, Gerunda GE, Depenni R, Zironi S. FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer. Br J Cancer. 2011;104:1079-1084. [PubMed] [DOI] |
| 15. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [PubMed] [DOI] |
| 16. | Sertic S, Pizzi S, Lazzaro F, Plevani P, Muzi-Falconi M. NER and DDR: classical music with new instruments. Cell Cycle. 2012;11:668-674. [PubMed] [DOI] |
| 17. | Bohanes P, Labonte MJ, Lenz HJ. A review of excision repair cross-complementation group 1 in colorectal cancer. Clin Colorectal Cancer. 2011;10:157-164. [PubMed] [DOI] |
| 18. | Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179-204. [PubMed] [DOI] |
| 19. | Li P, Fang YJ, Li F, Ou QJ, Chen G, Ma G. ERCC1, defective mismatch repair status as predictive biomarkers of survival for stage III colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. Br J Cancer. 2013;108:1238-1244. [PubMed] [DOI] |
| 20. | Kim KH, Kwon HC, Oh SY, Kim SH, Lee S, Kwon KA, Jang JS, Kim MC, Kim SJ, Kim HJ. Clinicopathologic significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers. 2011;16:74-82. [PubMed] [DOI] |
| 21. | Li HY, Ge X, Huang GM, Li KY, Zhao JQ, Yu XM, Bi WS, Wang YL. GSTP1, ERCC1 and ERCC2 polymorphisms, expression and clinical outcome of oxaliplatin-based adjuvant chemotherapy in colorectal cancer in Chinese population. Asian Pac J Cancer Prev. 2012;13:3465-3469. [PubMed] |
| 22. | Kim SH, Kwon HC, Oh SY, Lee DM, Lee S, Lee JH, Roh MS, Kim DC, Park KJ, Choi HJ. Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase pi for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am. J Clin Oncol. 2009;32:38-43. [PubMed] [DOI] |
| 23. | Subbarayan PR, Lee K, Ardalan B. Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant colorectal cancer cell line HT29 In Vitro re-sensitizing cells to 5-FU. Anticancer Res. 2010;30:1157-1162. [PubMed] |
| 24. | Ozasa H, Oguri T, Uemura T, Miyazaki M, Maeno K, Sato S, Ueda R. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010;101:161-166. [PubMed] [DOI] |
| 25. | Ceppi P, Rapa I, Lo Iacono M, Righi L, Giorcelli J, Pautasso M, Billè A, Ardissone F, Papotti M, Scagliotti GV. Expression and pharmacological inhibition of thymidylate synthase and Src kinase in nonsmall cell lung cancer. Int J Cancer. 2012;130:1777-1786. [PubMed] [DOI] |
| 26. | Lee KH, Hur HS, Im SA, Lee J, Kim HP, Yoon YK, Han SW, Song SH, Oh DY, Kim TY. RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase. Cancer Lett. 2010;299:22-28. [PubMed] [DOI] |
| 27. | Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int J Cancer. 2010;126:631-639. [PubMed] [DOI] |
| 28. | Kadota K, Huang CL, Liu D, Yokomise H, Haba R, Wada H. Combined therapy with a thymidylate synthase-inhibiting vector and S-1 has effective antitumor activity against 5-FU-resistant tumors. Int J Oncol. 2011;38:355-363. [PubMed] [DOI] |
| 29. | Yeh CN, Jung SM, Chen TW, Hwang TL, Jan YY, Chen MF. Expression of thymidylate synthase determines the response of gastric cancer patients undergoing gastrectomy to 5-fluorouracil-based adjuvant chemotherapy. Langenbecks Arch Surg. 2010;395:217-225. [PubMed] [DOI] |
| 30. | Temmink OH, Bijnsdorp IV, Prins HJ, Losekoot N, Adema AD, Smid K, Honeywell RJ, Ylstra B, Eijk PP, Fukushima M. Trifluorothymidine resistance is associated with decreased thymidine kinase and equilibrative nucleoside transporter expression or increased secretory phospholipase A2. Mol Cancer Ther. 2010;9:1047-1057. [PubMed] [DOI] |