修回日期: 2013-09-24
接受日期: 2013-11-06
在线出版日期: 2013-12-18
目的: 探讨内镜下支架置入联合新辅助化疗治疗结直肠癌恶性梗阻的临床效果.
方法: 回顾分析辽宁省人民医院普外科2003-2008年收治的结直肠癌恶性肠梗阻患者75例, 按治疗方案将患者分为对照组(n = 30), 采用传统方法治疗; 支架组(n = 30), 采用自膨式金属支架治疗; 支架化疗组(n = 15)采用自膨式金属支架联合新辅助化疗治疗.
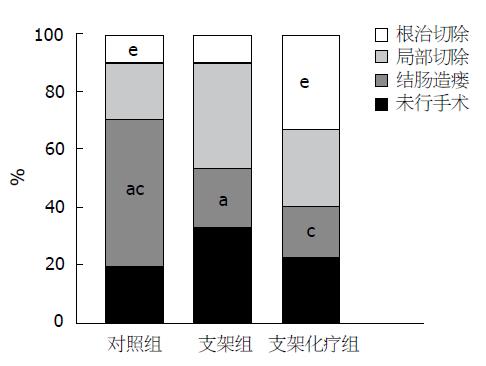
结果: 在手术治疗的患者中, 对照组实施结肠造瘘率高达62.5%, 明显高于支架组30.0%(χ2 = 4.619, P<0.05)及支架化疗组25.0%(χ2 = 4.500, P<0.05); 对照组肿瘤切除率达37.5%, 明显低于支架组70.0%(χ2 = 4.619, P<0.05)及支架化疗组75.0%(χ2 = 4.500, P<0.05); 其中实施根治性手术的患者中, 支架化疗组高达41.7%, 明显高于对照组12.5%(χ2 = 3.938, P<0.05), 对照组与支架组根治手术切除率比较无统计学意义(χ2 = 0.059, P>0.05); 支架化疗组5年生存率达26.7%, 明显高于对照组3.3%(χ2 = 5.513, P<0.05), 对照组与支架组比较无统计学意义(χ2 = 1.071, P>0.05).
结论: 内镜下金属支架置入联合新辅助化疗可以有效地提高晚期结直肠癌的手术切除率、根治率, 延长生存时间, 值得临床推广.
核心提示: 支架置入为恶性肠梗阻患者争取了宝贵治疗筹备时间, 而新辅助化疗则使部分不可切除肿瘤变为可切除肿瘤, 明显提高了肿瘤切除率及根治率、降低了肠造瘘率, 5年生存率显著提高, 为结直肠癌恶性肠梗阻患者提供了新的治疗途径.
引文著录: 吴洁, 荣大庆, 柳青峰, 耿宣, 张志强, 董齐, 王燕庆. 内镜下支架置入联合新辅助化疗治疗结直肠癌恶性梗阻. 世界华人消化杂志 2013; 21(35): 4056-4059
Revised: September 24, 2013
Accepted: November 6, 2013
Published online: December 18, 2013
AIM: To investigate the clinical effect of endoscopic stenting combined with neoadjuvant chemotherapy in the treatment of malignant colorectal obstruction.
METHODS: A retrospective analysis of 75 malignant colorectal obstruction patients who were treated at Liaoning Provincial People's Hospital between 2003 and 2008 was performed. The patients were divided into three groups, a control group (n = 30) treated using traditional methods, a stent placement group (n = 30) treated using self-expanding metal stents, and a stent placement plus chemotherapy group (n = 15) treated using self-expanding metal stents in combination with neoadjuvant chemotherapy.
RESULTS: The percentage of surgical patients undergoing colostomy was significantly higher in the control group than in the stent placement group and the stent placement plus chemotherapy group (62.5% vs 30.0%, 25.0%, χ2 = 4.619, 4.500, both P < 0.05). The tumor resection rate was significantly lower in the control group than in the stent placement group and the stent placement plus chemotherapy group (37.5% vs 70.0%, 75.0%, χ2 = 4.619, 4.500, both P < 0.05). The radical surgery rate was significantly higher in the stent placement plus chemotherapy than in the control group (41.7% vs 12.5%, χ2 = 3.938, P < 0.05), but showed no significant difference between the control group and stent placement group (χ2 = 0.059, P > 0.05). The five-year survival rate was significantly higher in the stent placement plus chemotherapy group than in the control group (26.7% vs 3.3%, χ2 = 5.513, P < 0.05), but showed no significant difference between the control group and stent placement group (χ2 = 1.071, P > 0.05).
CONCLUSION: Endoscopic stenting combined with neoadjuvant chemotherapy can effectively improve tumor resection rate and radical surgery rate and prolong survival time in patients with advanced colorectal cancer.
- Citation: Wu J, Rong DQ, Liu QF, Geng X, Zhang ZQ, Dong Q, Wang YQ. Endoscopic stenting combined with neoadjuvant chemotherapy for treatment of malignant colorectal obstruction. Shijie Huaren Xiaohua Zazhi 2013; 21(35): 4056-4059
- URL: https://www.wjgnet.com/1009-3079/full/v21/i35/4056.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v21.i35.4056
急性肠梗阻(acute ileus)是外科常见的急腹症, 85%的低位梗阻是由结直肠癌(colorectal cancer)引起, 而15%-20%的结直肠癌患者是在治疗肠梗阻的过程中发现的. 对于恶性肠梗阻(malignant intestinal obstruction)传统常需急诊手术治疗, 由于患者常伴有脱水、电解质紊乱、肠黏膜损伤、腹腔高压, 因此急诊手术风险巨大, 死亡率高达12%(择期手术死亡率约3.5%), 实施永久的结肠造口术患者也高达40%[1,2].
1991年Tirosh等[3]和Cirocchi等[4]首先报道了使用金属支架作为直肠癌的姑息治疗手段并获得满意的疗效. 支架置入操作简便, 患者耐受性好, 肠梗阻症状能够迅速改善, 且能有效地提高肠吻合率、降低吻合口瘘发生率. 随着内镜的发展, 支架置入成为了治疗恶性肠梗阻的重要手段.
然而支架介入只是一种姑息治疗方法, 并不能提高手术切除率、降低肝转移发生率, 也不能延长患者生存时间[5]. 我院通过内镜下支架置入联合新辅助化疗(neoadjuvant chemotherapy)治疗结直肠癌恶性梗阻取得了良好的疗效, 现报告如下.
回顾分析辽宁省人民医院普外科2003-2008年收治的结直肠癌恶性肠梗阻患者75例, 按治疗方案将患者分为对照组、支架组、支架化疗组. 病例选择标准: 有腹痛、腹胀等肠梗阻症状, 肠镜活检病理诊断为结直肠癌,临床分型在ⅢB以上. 排除标准: 患者病情严重不能耐受结肠镜检查者, 包括严重心脑血管疾患、急腹症、生命体征不平稳等; 神志异常不能配合检查者, 有结肠镜禁忌症者.
电子内镜: 富士400电子十二指肠镜系统, 德国西门子公司医用X射线系统, 美国bostorn公司生产的镍钛金属肠道支架、黄斑马导丝.
对照组(n = 30): 男20例, 女10例. 年龄46-78岁, 65.2岁±8.6岁, 所有患者入院后给予胃肠减压, 灌肠导泻等非手术治疗, 如肠道通畅, 则按病情择期手术或行化疗、支持治疗; 如肠梗阻无缓解则急诊手术行开腹肿瘤切除或肠造瘘术. 支架组(n = 30): 男16例, 女14例. 年龄54-80岁, 68.5岁±10.2岁, 所有患者入院确诊后均行结肠镜下内支架置入术: 结肠镜到达病变部位后, 在X线引导下将导丝放置到狭窄近端, 注入造影剂观察狭窄程度及长度, 选择支架, 在内镜直视和X线引导下将自膨式金属支架(self expanding metallic stent, SEMS)释放于狭窄部位, 两端超过病变部位2 cm左右. 支架置入后如肠道通畅, 则按病情择期手术或行化疗、支持治疗; 如肠梗阻无缓解则急诊手术行开腹肿瘤切除或肠造瘘术. 支架化疗组(n = 15): 男9例, 女6例. 年龄52-74岁, 67.8岁±12.5岁, 所有患者入院确诊后均行结肠镜下内支架置入术(方法同上); 如肠梗阻无缓解则急诊手术行开腹肿瘤切除或肠造瘘术; 如支架置入肠梗阻症状缓解, 给予mFOLFOX6方案新辅助化疗. mFOLFOX6方案: 奥沙利铂85 mg/m2静脉滴注2 h, 第1天; LV400 mg/m2静脉滴注2 h, 第1天: 5-FU mg/m2静脉推注, 第1天, 然后1200 mg/(m2·d)×2 d持续静脉滴注(总量2400 mg/m2, 滴注46-48 h)每2 wk重复. 新辅助化疗患者每月综合评估, 如不可切除肿瘤变为可切除肿瘤, 则择期行开腹手术切除肿瘤及转移灶[6-8].
统计学处理 所有数据均以mean±SD表示, 采用χ2检验分析方法, 经SPSS13.0统计分析软件对数据进行处理. P<0.05为差异具有统计学意义.
恶性肠梗阻患者由于肿瘤的不断进展, 通过非手术治疗使肠道再通畅的几率较低, 常需手术治疗; 本实验对照组20%患者行非手术治疗, 80%急诊行手术治疗(表1).
| 分组 | n | 平均年龄(岁) | 未手术 | 手术 | 手术治疗方式 | 5年生存例数 | ||
| 结肠造瘘 | 局部切除 | 根治切除 | ||||||
| 对照组 | 30 | 65.2±8.6 | 6(20.0) | 24(80.0) | 15(62.5) | 6(25.0) | 3(12.5) | 1(3.3) |
| 支架组 | 30 | 68.5±10.2 | 10(33.3) | 20(66.7) | 6(30.0) | 11(55.0) | 3(15) | 3(10.0) |
| 支架化疗组 | 15 | 67.8±12.5 | 3(20.0) | 12(80.0) | 3(25.0) | 4(33.3) | 5(41.7) | 4(26.7) |
在手术治疗的患者中, 对照组实施结肠造瘘率高达62.5%, 明显高于支架组30.0%(χ2 = 4.619, P<0.05)及支架化疗组25.0%(χ2 = 4.500, P<0.05); 对照组肿瘤切除率达37.5%, 明显低于支架组70.0%(χ2 = 4.619, P<0.05)及支架化疗组75.0%(χ2 = 4.500, P<0.05); 其中实施根治性手术的患者中, 支架化疗组高达41.7%, 明显高于对照组12.5%(χ2 = 3.938, P<0.05), 对照组与支架组根治手术切除率比较无统计学意义(χ2 = 0.059, P>0.05); 支架化疗组5年生存率达26.7%, 明显高于对照组3.3%(χ2 = 5.513, P<0.05), 对照组与支架组比较无统计学意义(χ2 = 1.071, P>0.05), (表1, 图1).
15%-20%的结直肠癌患者会发生恶性肠梗阻, 且当患者于急诊就诊时往往身体状况较差, 有研究表明, 梗阻是一个重要的、独立的不良预后因素, 可导致急诊手术的切除率低, 肠造口率高, 死亡率则高达12%. 目前利用SEMS替代恶性肠梗阻急诊手术正在被广泛接受, 并逐渐成为第一线的姑息性治疗方案. 这种无开腹创伤、低操作风险的方法能有效地缓解梗阻症状, 为那些有潜在可治愈的患者提供了术前充分评估、准备、支持的时间[9,10].
支架置入对恶性肠梗阻的早期治疗效果是毋庸置疑的, 但对其是否会损害危重患者的免疫系统、增加并发症的风险、增加可治愈肿瘤扩散的危险性等方面还存在争议; 且支架置入虽降低了肠造口率、吻合口瘘发生率、改善了生存质量, 但其对于肿瘤的切除率、肝转移发生率、患者的生存时间并没有显著的改善[11], 本实验也证实, 对照组与支架组比较, 手术根治率及5年生存率无统计学意义(P>0.05).
发生恶性肠梗阻的结直肠癌患者绝大部分都是肿瘤晚期, 局部浸润及肝转移发生率较高, 而手术切除肿瘤是延长患者生存时间最有效的办法, 行根治性手术的患者5年生存率为25%-40%, 未行手术切除或不可切除的患者5年生存率仅为2%-8%, 但适合手术切除的患者仅占10%-20%. 近年来国际上开始出现并提倡新辅助化疗来降低肿瘤的临床分期, 使一些不能切除的病症变为可能, 有研究表明新辅助化疗可使晚期结直肠癌切除率提高到30%[12,13].
NCCN指南2011版指出, 转移性结直肠癌确诊时大多数属于不可切除. 然而, 对那些转移瘤仅局限于肝脏并且累及重要结构而不可切除患者, 越来越倾向于考虑尝试使用术前化疗来缩小转移瘤体积以便尝试将其转化为可切除.
术前化疗相对于术后化疗的潜在优点还包括: 及早治疗微小转移灶; 判断肿瘤对化疗的反应, 有助于制定术后治疗计划; 对那些早期进展的患者可以避免局部治疗.
我们对结直肠癌恶性肠梗阻的患者, 置入支架后给予FOLFOX方案新辅助化疗, 取得了良好的临床效果. 支架组及支架化疗组实施结肠造瘘率明显低于对照组、肿瘤切除率明显高于对照组; 支架化疗组实施根治性手术的患者达41.7%明显高于对照组(P<0.05)、5年生存率达26.7%, 也明显高于对照组(P<0.05).
支架置入为恶性肠梗阻患者争取了宝贵治疗筹备时间, 而新辅助化疗则使部分不可切除肿瘤变为可切除肿瘤, 提高了患者的生存率. 随着结直肠癌发病率逐步提高, 新的治疗方法不断出现, 支架置入联合新辅助化疗为结直肠癌恶性肠梗阻患者提供了新的治疗途径, 而治疗前评估, 如何减轻化疗不良反应及新辅助化疗后的手术时间上还需要进一步的研究[14,15].
急性肠梗阻中85%的低位梗阻是由结直肠癌引起, 对于恶性肠梗阻传统常需急诊手术治疗, 由于患者常伴有脱水、电解质紊乱、肠黏膜损伤、腹腔高压, 因此急诊手术风险巨大, 死亡率高达12%, 实施永久的结肠造口术患者也高达40%.
杜群, 副研究员, 广州中医药大学脾胃研究所药理室
近年来研究较多的肝细胞材料为猪肝细胞和肿瘤源性肝细胞株(C3A、HepG2等), 但动物肝细胞存在发生免疫反应及传播动物源性病毒的危险, 肿瘤源性肝细胞株分化程度低、生物学功能低下且在临床应用中存在细胞逃逸进入患者体内引起肿瘤的危险, 限制了临床的广泛应用.
NCCN指南2011版指出, 转移性结直肠癌确诊时大多数属于不可切除. 然而, 对那些转移瘤仅局限于肝脏并且累及重要结构而不可切除患者, 越来越倾向于考虑尝试使用术前化疗来缩小转移瘤体积以便尝试将其转化为可切除.
本文目标基本明确, 设计合理, 实验数据可靠, 具有一定的创新性, 研究有一定的科学意义.
编辑: 田滢 电编:鲁亚静
| 1. | Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106:2174-2180. [PubMed] [DOI] |
| 2. | Bonfante P, D'Ambra L, Berti S, Falco E, Cristoni MV, Briglia R. Managing acute colorectal obstruction by "bridge stenting" to laparoscopic surgery: Our experience. World J Gastrointest Surg. 2012;4:289-295. [PubMed] [DOI] |
| 3. | Tirosh D, Perry Z, Walfisch S, Rozental A, Fich A, Krugliak P, Mizrahi S, Kirshtein B. Endoscopic self-expanding metal stents for acute colonic obstruction. Am Surg. 2013;79:30-34. [PubMed] |
| 4. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [PubMed] [DOI] |
| 5. | Ho KS, Quah HM, Lim JF, Tang CL, Eu KW. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis. 2012;27:355-362. [PubMed] [DOI] |
| 6. | Shao YC, Chang YY, Lin JK, Lin CC, Wang HS, Yang SH, Jiang JK, Lan YT, Lin TC, Li AF. Neoadjuvant chemotherapy can improve outcome of colorectal cancer patients with unresectable metastasis. Int J Colorectal Dis. 2013;28:1359-1365. [PubMed] [DOI] |
| 7. | Yao HW, Xiu DR, Fu W, Yuan J, Jiang B, Wang DC, Ma CL, Yuan CH, Sun T, Ma LW. [A clinical study on multi-disciplinary team and surgery for resectable colorectal cancer with liver metastases]. Zhonghua Waike Zazhi. 2012;50:961-965. [PubMed] |
| 8. | Fontana E, Pucci F, Camisa R, Bui S, Galdy S, Leonardi F, Negri FV, Anselmi E, Losardo PL, Roncoroni L. Long-term results of preoperative 5-fluorouracil-oxaliplatin chemoradiation therapy in locally advanced rectal cancer. Anticancer Res. 2013;33:725-730. [PubMed] |
| 9. | Fiori E, Lamazza A, Schillaci A, Femia S, Demasi E, Decesare A, Sterpetti AV. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg. 2012;204:321-326. [PubMed] [DOI] |
| 10. | Angenete E, Asplund D, Bergström M, Park PO. Stenting for colorectal cancer obstruction compared to surgery--a study of consecutive patients in a single institution. Int J Colorectal Dis. 2012;27:665-670. [PubMed] [DOI] |
| 11. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [PubMed] [DOI] |
| 13. | Ayez N, Burger JW, van der Pool AE, Eggermont AM, Grunhagen DJ, de Wilt JH, Verhoef C. Long-term results of the "liver first" approach in patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2013;56:281-287. [PubMed] [DOI] |
| 14. | Hermanek P, Merkel S, Hohenberger W. Prognosis of rectal carcinoma after multimodal treatment: ypTNM classification and tumor regression grading are essential. Anticancer Res. 2013;33:559-566. [PubMed] |
| 15. | Lu QY, Zhao AL, Deng W, Li ZW, Shen L. Hepatic histopathology and postoperative outcome after preoperative chemotherapy for Chinese patients with colorectal liver metastases. World J Gastrointest Surg. 2013;5:30-36. [PubMed] [DOI] |